Fluorinated peptide biomaterials
- PMID: 34541446
- PMCID: PMC8448251
- DOI: 10.1002/pep2.24184
Fluorinated peptide biomaterials
Abstract
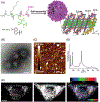
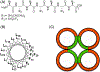
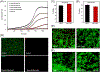
Fluorinated compounds, while rarely used by nature, are emerging as fundamental ingredients in biomedical research, with applications in drug discovery, metabolomics, biospectroscopy, and, as the focus of this review, peptide/protein engineering. Leveraging the fluorous effect to direct peptide assembly has evolved an entirely new class of organofluorine building blocks from which unique and bioactive materials can be constructed. Here, we discuss three distinct peptide fluorination strategies used to design and induce peptide assembly into nano-, micro-, and macrosupramolecular states that potentiate high-ordered organization into material scaffolds. These fluorine-tailored peptide assemblies employ the unique fluorous environment to boost biofunctionality for a broad range of applications, from drug delivery to antibacterial coatings. This review provides foundational tactics for peptide fluorination and discusses the utility of these fluorous-directed hierarchical structures as material platforms in diverse biomedical applications.
Keywords: biomaterials; fluorine biochemistry; peptides; supramolecular assembly.
Conflict of interest statement
CONFLICT OF INTEREST The authors declare they have no conflict of interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
