The role of immunotherapy for management of advanced thymic epithelial tumors: a narrative review
- PMID: 34541456
- PMCID: PMC8445513
- DOI: 10.21037/med-20-62
The role of immunotherapy for management of advanced thymic epithelial tumors: a narrative review
Abstract
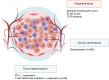
The emergence of immunotherapy as a modern pillar of cancer treatment has changed the treatment landscape for various cancers. Immune checkpoint inhibitors directed at programed death-1 (PD-1) or its ligand (PD-L1), in particular, have found widespread clinical applications and have resulted in durable responses and an improvement in survival of patients with advanced or metastatic disease. Tumor cell PD-L1 expression and tumor mutation burden (TMB) are biomarkers of response and efforts are underway to identify other biomarkers that might predict benefit with these drugs. Most patients tolerate immunotherapy well, although a subset of patients develop immune-mediated toxicity due to excessive immune stimulation. Thymic epithelial tumors (TETs) have a unique biology which can predispose to development of autoimmune paraneoplastic disease, especially in patients with thymoma. Due to defects in immunological self-tolerance, the use of immunotherapy in TET patients is associated with an increased risk of immune-mediated adverse events, which can be potentially life-threatening. Development of biomarkers of response and toxicity is particularly important for the treatment of TETs since it is important to identify patients who might benefit from treatment and be at low risk for development of severe immune toxicity. The use of immunotherapy in patients with autoimmune disorders and those who have previously experienced immune-mediated toxicity is currently an area of active research. Various risk mitigation strategies are under evaluation in prospective clinical trials, including trials of immune checkpoint inhibitors in patients with thymic cancers.
Keywords: Thymic tumors; clinical research; immunotherapy; systemic therapy.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-62). The series “Dedicated to the 10th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2019)” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
