Managing Adverse Events Associated with Dinutuximab Beta Treatment in Patients with High-Risk Neuroblastoma: Practical Guidance
- PMID: 34541620
- PMCID: PMC8563639
- DOI: 10.1007/s40272-021-00469-9
Managing Adverse Events Associated with Dinutuximab Beta Treatment in Patients with High-Risk Neuroblastoma: Practical Guidance
Abstract
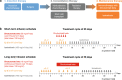
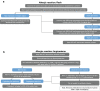
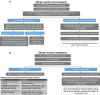
Neuroblastoma is the most common extracranial solid tumour in children, accounting for 15% of all paediatric cancer deaths. High-risk neuroblastoma is a particularly challenging-to-treat form of disease that requires multimodality treatment, consisting of chemotherapy, surgery, high-dose chemotherapy with autologous haematopoietic stem cell rescue, radiotherapy and differentiation therapy. However, despite intense multimodal treatment regimens, the prognosis for this patient population remains poor. In recent years, immunotherapy with anti-disialoganglioside 2 (anti-GD2) antibodies was found to improve survival rates for patients with high-risk neuroblastoma. Based on studies led by the SIOPEN (International Society of Paediatric Oncology European Neuroblastoma) group, the anti-GD2 antibody dinutuximab beta was approved for use in high-risk neuroblastoma by the European Medicines Agency and has been implemented into the standard of care in many countries across Europe. However, immunotherapy with dinutuximab beta is associated with a number of adverse events that may be challenging for clinicians, such as pain, fever, hypersensitivity reactions and capillary leak syndrome. While these adverse events are considered manageable, there are currently no formal guidelines to support clinicians with their management. The aim of this article is to discuss the management of the most common adverse events encountered in clinical practice and to provide practical guidance to assist clinicians in minimising toxicity associated with dinutuximab beta.
© 2021. The Author(s).
Conflict of interest statement
GB, FB, BB, ASD, FH, CM, DR, PMR, AW and MvN received compensation for their role as a consultant/advisor at the EUSA Pharma Advisory Board meeting that has led to the subsequent development of this publication. GB has also received educational funding from EUSA Pharma. AB declares that she has no conflicts of interest that might be relevant to the contents of this article. FB had a consultant or advisory role and support for attending symposia from EUSA Pharma. ASD has received support for attending symposia from EUSA Pharma. FH has received support for attending symposia from EUSA Pharma. DR received support for attending symposia from EUSA Pharma. PMR had a consultant or advisory role and support for attending symposia from EUSA Pharma. AW has received support for attending symposia from EUSA Pharma and has a consulting agreement with EUSA Pharma.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

