Mechanical ventilation in COVID-19: A physiological perspective
- PMID: 34541721
- PMCID: PMC8667647
- DOI: 10.1113/EP089400
Mechanical ventilation in COVID-19: A physiological perspective
Abstract
New findings: What is the topic of this review? This review presents the fundamental concepts of respiratory physiology and pathophysiology, with particular reference to lung mechanics and the pulmonary phenotype associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and subsequent coronavirus disease 2019 (COVID-19) pneumonia. What advances does it highlight? The review provides a critical summary of the main physiological aspects to be considered for safe and effective mechanical ventilation in patients with severe COVID-19 in the intensive care unit.
Abstract: Severe respiratory failure from coronavirus disease 2019 (COVID-19) pneumonia not responding to non-invasive respiratory support requires mechanical ventilation. Although ventilation can be a life-saving therapy, it can cause further lung injury if airway pressure and flow and their timing are not tailored to the respiratory system mechanics of the individual patient. The pathophysiology of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can lead to a pattern of lung injury in patients with severe COVID-19 pneumonia typically associated with two distinct phenotypes, along a temporal and pathophysiological continuum, characterized by different levels of elastance, ventilation-to-perfusion ratio, right-to-left shunt, lung weight and recruitability. Understanding the underlying pathophysiology, duration of symptoms, radiological characteristics and lung mechanics at the individual patient level is crucial for the appropriate choice of mechanical ventilation settings to optimize gas exchange and prevent further lung injury. By critical analysis of the literature, we propose fundamental physiological and mechanical criteria for the selection of ventilation settings for COVID-19 patients in intensive care units. In particular, the choice of tidal volume should be based on obtaining a driving pressure < 14 cmH2 O, ensuring the avoidance of hypoventilation in patients with preserved compliance and of excessive strain in patients with smaller lung volumes and lower lung compliance. The level of positive end-expiratory pressure (PEEP) should be informed by the measurement of the potential for lung recruitability, where patients with greater recruitability potential may benefit from higher PEEP levels. Prone positioning is often beneficial and should be considered early. The rationale for the proposed mechanical ventilation settings criteria is presented and discussed.
Keywords: COVID-19; SARS-CoV-2; artificial; critical care; physiology; respiration; respiratory; respiratory distress syndrome.
© 2021 The Authors. Experimental Physiology © 2021 The Physiological Society.
Conflict of interest statement
None declared.
Figures
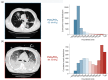
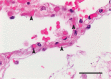

References
-
- Ackermann, M. , Verleden, S. E. , Kuehnel, M. , Haverich, A. , Welte, T. , Laenger, F. , Vanstapel, A. , Werlein, C. , Stark, H. , Tzankov, A. , Li, W. W. , Li, V. W. , Mentzer, S. J. , & Jonigk, D. (2020). Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. New England Journal of Medicine, 383, 120–128. 10.1056/NEJMoa2015432 - DOI - PMC - PubMed
-
- Amato, M. B. P. , Meade, M. O. , Slutsky, A. S. , Brochard, L. , Costa, E. L. V. , Schoenfeld, D. A. , Stewart, T. E. , Briel, M. , Talmor, D. , Mercat, A. , Richard, J.‐C. M. , Carvalho, C. R. R. , & Brower, R. G. (2015). Driving pressure and survival in the acute respiratory distress syndrome. New England Journal of Medicine, 372, 747–755. 10.1056/NEJMsa1410639 - DOI - PubMed
-
- Ball, L. , Robba, C. , Maiello, L. , Herrmann, J. , Gerard, S. E. , Xin, Y. , Battaglini, D. , Brunetti, I. , Minetti, G. , & Seitun, S. (2021). Computed tomography assessment of PEEP‐induced alveolar recruitment in patients with severe COVID‐19 pneumonia. Critical Care, 25, 81. 10.1186/s13054-021-03477-w - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

