Single- and Two-Stage, Closed-Tube, Point-of-Care, Molecular Detection of SARS-CoV-2
- PMID: 34541844
- PMCID: PMC8482318
- DOI: 10.1021/acs.analchem.1c03016
Single- and Two-Stage, Closed-Tube, Point-of-Care, Molecular Detection of SARS-CoV-2
Abstract
Short of a vaccine, frequent and rapid testing, preferably at home, is the most effective strategy to contain the COVID-19 pandemic. Herein, we report on single-stage and two-stage molecular diagnostic tests that can be carried out with simple or no instrumentation. Our single-stage amplification is reverse transcription-loop mediated isothermal amplification (RT-LAMP) with custom-designed primers targeting the ORF1ab and the N gene regions of the virus genome. Our new two-stage amplification, dubbed Penn-RAMP, comprises recombinase isothermal amplification (RT-RPA) as its first stage and LAMP as its second stage. We compared various sample preparation strategies aimed at deactivating the virus while preserving its RNA and tested contrived and patient samples, consisting of nasopharyngeal swabs, oropharyngeal swabs, and saliva. Amplicons were detected either in real time with fluorescent intercalating dye or after amplification with the intercalating colorimetric dye LCV, which is insensitive to sample's PH. Our single RT-LAMP tests can be carried out instrumentation-free. To enable concurrent testing of multiple samples, we developed an inexpensive heat block that supports both the single-stage and two-stage amplification. Our RT-LAMP and Penn-RAMP assays have, respectively, analytical sensitivities of 50 and 5 virions/reaction. Both our single- and two-stage assays have successfully detected SARS-CoV-2 in patients with viral loads corresponding to the reverse transcription-quantitative polymerase chain reaction (RT-qPCR) threshold cycle smaller than 32 while operating with minimally processed samples, without nucleic acid isolation. Penn-RAMP provides a 10-fold better sensitivity than RT-LAMP and does not need thermal cycling like PCR assays. All reagents are amenable to dry, refrigeration-free storage. The SARS-CoV-2 test described herein is suitable for screening at home, at the point of need, and in resource-poor settings.
Figures
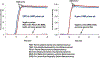

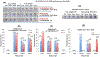

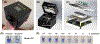
Update of
-
A Single and Two-Stage, Closed-Tube, Molecular Test for the 2019 Novel Coronavirus (COVID-19) at Home, Clinic, and Points of Entry.ChemRxiv [Preprint]. 2020 Feb 19. doi: 10.26434/chemrxiv.11860137.v1. ChemRxiv. 2020. Update in: Anal Chem. 2021 Sep 28;93(38):13063-13071. doi: 10.1021/acs.analchem.1c03016. PMID: 32511284 Free PMC article. Updated. Preprint.
Similar articles
-
A Multiplex and Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification Assay for Sensitive and Rapid Detection of Novel SARS-CoV-2.Front Cell Infect Microbiol. 2021 Jun 29;11:653616. doi: 10.3389/fcimb.2021.653616. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34268131 Free PMC article.
-
Rapid and Extraction-Free Detection of SARS-CoV-2 from Saliva by Colorimetric Reverse-Transcription Loop-Mediated Isothermal Amplification.Clin Chem. 2021 Jan 30;67(2):415-424. doi: 10.1093/clinchem/hvaa267. Clin Chem. 2021. PMID: 33098427 Free PMC article.
-
Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection.mSphere. 2020 Aug 26;5(4):e00808-20. doi: 10.1128/mSphere.00808-20. mSphere. 2020. PMID: 32848011 Free PMC article.
-
Reverse Transcriptase Loop Mediated Isothermal Amplification (RT-LAMP) for COVID-19 diagnosis: a systematic review and meta-analysis.Pathog Glob Health. 2021 Jul;115(5):281-291. doi: 10.1080/20477724.2021.1933335. Epub 2021 Jun 4. Pathog Glob Health. 2021. PMID: 34086539 Free PMC article.
-
COVID-19 Diagnosis: Current and Future Techniques.Int J Biol Macromol. 2021 Dec 15;193(Pt B):1835-1844. doi: 10.1016/j.ijbiomac.2021.11.016. Epub 2021 Nov 12. Int J Biol Macromol. 2021. PMID: 34774862 Free PMC article. Review.
Cited by
-
SARS-CoV-2 Diagnostics Based on Nucleic Acids Amplification: From Fundamental Concepts to Applications and Beyond.Front Cell Infect Microbiol. 2022 Mar 23;12:799678. doi: 10.3389/fcimb.2022.799678. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35402302 Free PMC article. Review.
-
Advancements and applications of loop-mediated isothermal amplification technology: a comprehensive overview.Front Microbiol. 2024 Jul 17;15:1406632. doi: 10.3389/fmicb.2024.1406632. eCollection 2024. Front Microbiol. 2024. PMID: 39091309 Free PMC article. Review.
-
A Novel Real-Time Reverse Transcription Loop-Mediated Isothermal Amplification Detection Platform: Application to Diagnosis of COVID-19.Front Bioeng Biotechnol. 2021 Oct 22;9:748746. doi: 10.3389/fbioe.2021.748746. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34746104 Free PMC article. Review.
-
Engineering Materials and Devices for the Prevention, Diagnosis, and Treatment of COVID-19 and Infectious Diseases.Nanomaterials (Basel). 2023 Aug 30;13(17):2455. doi: 10.3390/nano13172455. Nanomaterials (Basel). 2023. PMID: 37686965 Free PMC article. Review.
-
Trends of respiratory virus detection in point-of-care testing: A review.Anal Chim Acta. 2023 Jul 11;1264:341283. doi: 10.1016/j.aca.2023.341283. Epub 2023 May 3. Anal Chim Acta. 2023. PMID: 37230728 Free PMC article. Review.
References
-
- Zeng ZQ; Chen DH; Tan WP; Qiu SY; Xu D; Liang HX; Chen MX; Li X; Lin ZS; Liu WK; Zhou R Epidemiology and Clinical Characteristics of Human Coronaviruses Oc43, 229e, Nl63, and Hku1: A Study of Hospitalized Children with Acute Respiratory Tract Infection in Guangzhou, China. Eur. J. Clin. Microbiol. Infect. Dis 2018, 37, 363–369. - PMC - PubMed
-
- WHO, Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). World Health Organization 2003. https://apps.who.int/iris/handle/10665/70863 (accessed July 17, 2021).
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

