Radioembolization With Chemotherapy for Colorectal Liver Metastases: A Randomized, Open-Label, International, Multicenter, Phase III Trial
- PMID: 34541864
- PMCID: PMC8660005
- DOI: 10.1200/JCO.21.01839
Radioembolization With Chemotherapy for Colorectal Liver Metastases: A Randomized, Open-Label, International, Multicenter, Phase III Trial
Abstract
Purpose: To study the impact of transarterial Yttrium-90 radioembolization (TARE) in combination with second-line systemic chemotherapy for colorectal liver metastases (CLM).
Methods: In this international, multicenter, open-label phase III trial, patients with CLM who progressed on oxaliplatin- or irinotecan-based first-line therapy were randomly assigned 1:1 to receive second-line chemotherapy with or without TARE. The two primary end points were progression-free survival (PFS) and hepatic PFS (hPFS), assessed by blinded independent central review. Random assignment was performed using a web- or voice-based system stratified by unilobar or bilobar disease, oxaliplatin- or irinotecan-based first-line chemotherapy, and KRAS mutation status.
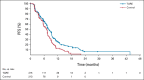
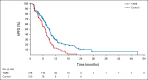
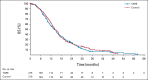
Results: Four hundred twenty-eight patients from 95 centers in North America, Europe, and Asia were randomly assigned to chemotherapy with or without TARE; this represents the intention-to-treat population and included 215 patients in the TARE plus chemotherapy group and 213 patients in the chemotherapy alone group. The hazard ratio (HR) for PFS was 0.69 (95% CI, 0.54 to 0.88; 1-sided P = .0013), with a median PFS of 8.0 (95% CI, 7.2 to 9.2) and 7.2 (95% CI, 5.7 to 7.6) months, respectively. The HR for hPFS was 0.59 (95% CI, 0.46 to 0.77; 1-sided P < .0001), with a median hPFS of 9.1 (95% CI, 7.8 to 9.7) and 7.2 (95% CI, 5.7 to 7.6) months, respectively. Objective response rates were 34.0% (95% CI, 28.0 to 40.5) and 21.1% (95% CI, 16.2 to 27.1; 1-sided P = .0019) for the TARE and chemotherapy groups, respectively. Median overall survival was 14.0 (95% CI, 11.8 to 15.5) and 14.4 months (95% CI, 12.8 to 16.4; 1-sided P = .7229) with a HR of 1.07 (95% CI, 0.86 to 1.32) for TARE and chemotherapy groups, respectively. Grade 3 adverse events were reported more frequently with TARE (68.4% v 49.3%). Both groups received full chemotherapy dose intensity.
Conclusion: The addition of TARE to systemic therapy for second-line CLM led to longer PFS and hPFS. Further subset analyses are needed to better define the ideal patient population that would benefit from TARE.
Trial registration: ClinicalTrials.gov NCT01483027.
Conflict of interest statement
Figures

Comment in
-
Transarterial Radioembolization in Patients With Unresectable Colorectal Cancer Liver Metastases.J Clin Oncol. 2021 Dec 10;39(35):3887-3889. doi: 10.1200/JCO.21.01993. Epub 2021 Sep 20. J Clin Oncol. 2021. PMID: 34541862 No abstract available.
References
-
- Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 - PubMed
-
- Uhlig J, Lukovic J, Dawson LA, et al. : Locoregional therapies for colorectal cancer liver metastases: Options beyond resection. Am Soc Clin Oncol Ed Book 41:133-146, 2021 - PubMed
-
- Salem R, Thurston KG: Radioembolization with 90Yttrium microspheres: A state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol 17:1251-1278, 2006 - PubMed
-
- Mulcahy MF, Lewandowski RJ, Ibrahim SM, et al. : Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer 115:1849-1858, 2009 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

