Morphological and Molecular Evaluation of the Tissue Repair following Nasal Septum Biopsy in a Sheep Model
- PMID: 34541930
- PMCID: PMC8804720
- DOI: 10.1177/19476035211046040
Morphological and Molecular Evaluation of the Tissue Repair following Nasal Septum Biopsy in a Sheep Model
Abstract
Objective: Nasal septal pathologies requiring surgical intervention are common in the population. Additionally, nasal chondrocytes are becoming an important cell source in cartilage tissue engineering strategies for the repair of articular cartilage lesions. These procedures damage the nasal septal cartilage whose healing potential is limited due to its avascular, aneural, and alymphatic nature. Despite the high incidence of various surgical interventions that affect septum cartilage, limited nasal cartilage repair characterizations have been performed to date.
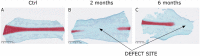
Methods: To evaluate the healing of the nasal septum cartilage perforation, a septal biopsy was performed in 14 sheep. Two and 6 months later, the tissue formed on the place of perforation was explanted and compared with the native tissue. Tissue morphology, protein and gene expression of explanted tissue was determined using histological, immunohistochemical and real-time quantitative polymerase chain reaction analysis.
Results: Tissue formed on the defect site, 2 and 6 months after the biopsy was characterized as mostly connective tissue with the presence of fibroblastic cells. This newly formed tissue contained no glycosaminoglycans and collagen type II but was positively stained for collagen type I. Cartilage-specific genes COL2, AGG, and COMP were significantly decreased in 2- and 6-month samples compared with the native nasal cartilage. Levels of COL1, COL4, and CRABP1 genes specific for perichondrium and connective tissue were higher in both test group samples in comparison with native cartilage.
Conclusions: Newly formed tissue was not cartilage but rather fibrous tissue suggesting the role of perichondrium and mucosa in tissue repair after nasal septum injury.
Keywords: biopsy; cartilage; healing; nasal chondrocytes; sheep.
Conflict of interest statement
Figures




Similar articles
-
Evaluation of Collagen Gel-Associated Human Nasal Septum-Derived Chondrocytes As a Clinically Applicable Injectable Therapeutic Agent for Cartilage Repair.Tissue Eng Regen Med. 2020 Jun;17(3):387-399. doi: 10.1007/s13770-020-00261-9. Epub 2020 May 12. Tissue Eng Regen Med. 2020. PMID: 32399775 Free PMC article.
-
Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial.Lancet. 2016 Oct 22;388(10055):1985-1994. doi: 10.1016/S0140-6736(16)31658-0. Lancet. 2016. PMID: 27789021
-
Marine collagen scaffolds for nasal cartilage repair: prevention of nasal septal perforations in a new orthotopic rat model using tissue engineering techniques.Tissue Eng Part A. 2013 Oct;19(19-20):2201-14. doi: 10.1089/ten.TEA.2012.0650. Epub 2013 Jun 5. Tissue Eng Part A. 2013. PMID: 23621795 Free PMC article.
-
Toward tissue-engineering of nasal cartilages.Acta Biomater. 2019 Apr 1;88:42-56. doi: 10.1016/j.actbio.2019.02.025. Epub 2019 Feb 19. Acta Biomater. 2019. PMID: 30794988 Review.
-
Challenges in Nasal Cartilage Tissue Engineering to Restore the Shape and Function of the Nose.Tissue Eng Part B Rev. 2024 Dec;30(6):581-595. doi: 10.1089/ten.TEB.2023.0326. Epub 2024 Apr 17. Tissue Eng Part B Rev. 2024. PMID: 38411533 Review.
Cited by
-
Hydrogel composite scaffolds achieve recruitment and chondrogenesis in cartilage tissue engineering applications.J Nanobiotechnology. 2022 Jan 6;20(1):25. doi: 10.1186/s12951-021-01230-7. J Nanobiotechnology. 2022. PMID: 34991615 Free PMC article.
-
LncRNA SNHG1 enhances cartilage regeneration by modulating chondrogenic differentiation and angiogenesis potentials of JBMMSCs via mitochondrial function regulation.Stem Cell Res Ther. 2024 Jun 18;15(1):177. doi: 10.1186/s13287-024-03793-2. Stem Cell Res Ther. 2024. PMID: 38886785 Free PMC article.
-
Properties of the Nasal Cartilage, from Development to Adulthood: A Scoping Review.Cartilage. 2022 Jan-Mar;13(1):19476035221087696. doi: 10.1177/19476035221087696. Cartilage. 2022. PMID: 35345900 Free PMC article.
References
-
- Park SS, Becker DG. Rhinological evaluation. In: Laws ER, Lanzino G, editors. Transsphenoidal surgery. WB Saunders; 2010:36-42.
-
- Ustünel I, Cayli S, Güney K, Celik-Ozenci C, Tanriöver G, Sahin Z, et al.. Immunohistochemical distribution patterns of collagen type II, chondroitin 4-sulfate, laminin and fibronectin in human nasal septal cartilage. Acta Histochem. 2003;105(2_suppl):109-14. doi:10.1078/0065-1281-00699 - DOI - PubMed
-
- Theocharis AD, Karamanos NK, Papageorgakopoulou N, Tsiganos CP, Theocharis DA. Isolation and characterization of matrix proteoglycans from human nasal cartilage. Compositional and structural comparison between normal and scoliotic tissues. Biochim Biophys Acta. 2002;1569(1-3):117-26. doi:10.1016/s0304-4165(01)00242-2 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

