Use and Safety of Immunotherapeutic Management of N-Methyl-d-Aspartate Receptor Antibody Encephalitis: A Meta-analysis
- PMID: 34542573
- PMCID: PMC8453367
- DOI: 10.1001/jamaneurol.2021.3188
Use and Safety of Immunotherapeutic Management of N-Methyl-d-Aspartate Receptor Antibody Encephalitis: A Meta-analysis
Abstract
Importance: Overall, immunotherapy has been shown to improve outcomes and reduce relapses in individuals with N-methyl-d-aspartate receptor (NMDAR) antibody encephalitis (NMDARE); however, the superiority of specific treatments and combinations remains unclear.
Objective: To map the use and safety of immunotherapies in individuals with NMDARE, identify early predictors of poor functional outcome and relapse, evaluate changes in immunotherapy use and disease outcome over the 14 years since first reports of NMDARE, and assess the Anti-NMDAR Encephalitis One-Year Functional Status (NEOS) score.
Data sources: Systematic search in PubMed from inception to January 1, 2019.
Study selection: Published articles including patients with NMDARE with positive NMDAR antibodies and available individual immunotherapy data.
Data extraction and synthesis: Individual patient data on immunotherapies, clinical characteristics at presentation, disease course, and final functional outcome (modified Rankin Scale [mRS] score) were entered into multivariable logistic regression models.
Main outcomes and measures: The planned study outcomes were functional outcome at 12 months from disease onset (good, mRS score of 0 to 2; poor, mRS score greater than 2) and monophasic course (absence of relapse at 24 months or later from onset).
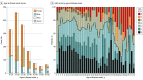
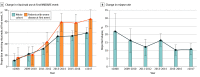
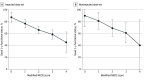
Results: Data from 1550 patients from 652 articles were evaluated. Of these, 1105 of 1508 (73.3%) were female and 707 of 1526 (46.3%) were 18 years or younger at disease onset. Factors at first event that were significantly associated with good functional outcome included adolescent age and first-line treatment with therapeutic apheresis, corticosteroids plus intravenous immunoglobulin (IVIG), or corticosteroids plus IVIG plus therapeutic apheresis. Factors significantly associated with poor functional outcome were age younger than 2 years or age of 65 years or older at onset, intensive care unit admission, extreme delta brush pattern on electroencephalography, lack of immunotherapy within the first 30 days of onset, and maintenance IVIG use for 6 months or more. Factors significantly associated with nonrelapsing disease were rituximab use or maintenance IVIG use for 6 months or more. Adolescent age at onset was significantly associated with relapsing disease. Rituximab use increased from 13.5% (52 of 384; 2007 to 2013) to 28.3% (311 of 1100; 2013 to 2019) (P < .001), concurrent with a falling relapse rate over the same period (22% [12 of 55] in 2008 and earlier; 10.9% [35 of 322] in 2017 and later; P = .006). Modified NEOS score (including 4 of 5 original NEOS items) was associated with probability of poor functional status at 1 year (20.1% [40 of 199] for a score of 0 to 1 points; 43.8% [77 of 176] for a score of 3 to 4 points; P = .05).
Conclusions and relevance: Factors influencing functional outcomes and relapse are different and need to be considered independently in development of evidence-based optimal management guidelines of patients with NMDARE.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

