Phenotype Bias Determines How Natural RNA Structures Occupy the Morphospace of All Possible Shapes
- PMID: 34542628
- PMCID: PMC8763027
- DOI: 10.1093/molbev/msab280
Phenotype Bias Determines How Natural RNA Structures Occupy the Morphospace of All Possible Shapes
Abstract
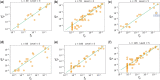
Morphospaces-representations of phenotypic characteristics-are often populated unevenly, leaving large parts unoccupied. Such patterns are typically ascribed to contingency, or else to natural selection disfavoring certain parts of the morphospace. The extent to which developmental bias, the tendency of certain phenotypes to preferentially appear as potential variation, also explains these patterns is hotly debated. Here we demonstrate quantitatively that developmental bias is the primary explanation for the occupation of the morphospace of RNA secondary structure (SS) shapes. Upon random mutations, some RNA SS shapes (the frequent ones) are much more likely to appear than others. By using the RNAshapes method to define coarse-grained SS classes, we can directly compare the frequencies that noncoding RNA SS shapes appear in the RNAcentral database to frequencies obtained upon a random sampling of sequences. We show that: 1) only the most frequent structures appear in nature; the vast majority of possible structures in the morphospace have not yet been explored; 2) remarkably small numbers of random sequences are needed to produce all the RNA SS shapes found in nature so far; and 3) perhaps most surprisingly, the natural frequencies are accurately predicted, over several orders of magnitude in variation, by the likelihood that structures appear upon a uniform random sampling of sequences. The ultimate cause of these patterns is not natural selection, but rather a strong phenotype bias in the RNA genotype-phenotype map, a type of developmental bias or "findability constraint," which limits evolutionary dynamics to a hugely reduced subset of structures that are easy to "find."
Keywords: RNA structure; evolution; morphospace; phenotype bias.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures


References
-
- Ahnert S, Johnston I, Fink T, Doye J, Louis A.. 2010. Self-assembly, modularity, and physical complexity. Phys Rev E Stat Nonlin Soft Matter Phys. 82(2 Pt 2):026117. - PubMed
-
- Arthur W. 2001. Developmental drive: an important determinant of the direction of phenotypic evolution. Evol Dev. 3(4):271–278. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

