Ocellar spatial vision in Myrmecia ants
- PMID: 34542631
- PMCID: PMC8546673
- DOI: 10.1242/jeb.242948
Ocellar spatial vision in Myrmecia ants
Abstract
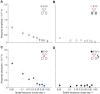
In addition to compound eyes, insects possess simple eyes known as ocelli. Input from the ocelli modulates optomotor responses, flight-time initiation, and phototactic responses - behaviours that are mediated predominantly by the compound eyes. In this study, using pattern electroretinography (pERG), we investigated the contribution of the compound eyes to ocellar spatial vision in the diurnal Australian bull ant Myrmecia tarsata by measuring the contrast sensitivity and spatial resolving power of the ocellar second-order neurons under various occlusion conditions. Furthermore, in four species of Myrmecia ants active at different times of the day, and in European honeybee Apis mellifera, we characterized the ocellar visual properties when both visual systems were available. Among the ants, we found that the time of activity had no significant effect on ocellar spatial vision. Comparing day-active ants and the honeybee, we did not find any significant effect of locomotion on ocellar spatial vision. In M. tarsata, when the compound eyes were occluded, the amplitude of the pERG signal from the ocelli was reduced 3 times compared with conditions when the compound eyes were available. The signal from the compound eyes maintained the maximum contrast sensitivity of the ocelli as 13 (7.7%), and the spatial resolving power as 0.29 cycles deg-1. We conclude that ocellar spatial vison improves significantly with input from the compound eyes, with a noticeably larger improvement in contrast sensitivity than in spatial resolving power.
Keywords: Bull ants; Contrast sensitivity; Flying; Honeybees; Pattern electroretinography; Spatial resolving power; Walking.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
-
- Barry, C. K. and Jander, R. (1968). Photoinhibitory function of the dorsal ocelli in the phototactic reaction of the migratory locust Locusta migratoria L. Nature 217, 675-677. 10.1038/217675a0 - DOI
-
- Bates, D., Mächler, M., Bolker, B. and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. 10.18637/jss.v067.i01 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

