The Safety of Agalsidase Alfa Enzyme Replacement Therapy in Canadian Patients with Fabry Disease Following Implementation of a Bioreactor Process
- PMID: 34542871
- PMCID: PMC8602602
- DOI: 10.1007/s40268-021-00361-4
The Safety of Agalsidase Alfa Enzyme Replacement Therapy in Canadian Patients with Fabry Disease Following Implementation of a Bioreactor Process
Abstract
Background and objective: Fabry disease, an X-linked lysosomal storage disorder characterized by absent or reduced alpha-galactosidase activity, is a lifelong disease that impairs patients' quality of life. Patients with Fabry disease have a considerably shortened lifespan, with mortality being mainly due to renal failure, cardiovascular disease, or cerebrovascular disease. Enzyme replacement therapy with agalsidase alfa has been shown to attenuate the renal, cardiovascular, and neuropathic disease progression associated with Fabry disease. The objective of this study was to investigate the safety of a new animal component-free version of agalsidase alfa.
Methods: A phase III/IV, open-label, single-arm, multicenter safety study was conducted in Canadian patients with Fabry disease between August 2011 and September 2017 as a regulatory requirement to assess the safety of agalsidase alfa produced using an animal component-free bioreactor process. Eligible patients had a documented diagnosis of Fabry disease and satisfied current Canadian guidelines for receiving enzyme replacement therapy for Fabry disease. Following treatment with animal component-free bioreactor-processed agalsidase alfa, treatment-emergent adverse events were monitored, and post hoc analyses of infusion-related reactions by antidrug antibody and neutralizing antibody statuses were conducted. The data were analyzed using descriptive statistics.
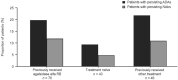
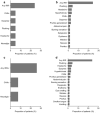
Results: A total of 167 patients (mean [standard deviation] age, 48.9 [14.8] years), including six pediatric patients (< 18 years of age), received at least one full or partial infusion of agalsidase alfa animal component-free. Fewer than 5% of treatment-emergent adverse events (212/4446) observed in 40 patients were reported as infusion-related reactions. Antidrug antibody and neutralizing antibody status did not affect the proportion of patients with infusion-related reactions. No clinically significant changes in vital signs were observed in patients over the course of the study.
Conclusions: Long-term treatment with bioreactor-produced agalsidase alfa animal component-free did not reveal new safety signals in this population of Canadian patients with Fabry disease. The treatment-emergent adverse event profile was consistent with the clinical manifestations of the disease and the known safety profile of roller bottle-produced agalsidase alfa.
Clinical trial registration: ClinicalTrials.gov identifier NCT01298141.
© 2021. The Author(s).
Conflict of interest statement
Dr. Khan reports grants and personal fees from Takeda, outside of the submitted work. Dr. Sirrs reports grants from and advisory board participation for Sanofi Genzyme, grants and travel support from Takeda, and grants and travel support from Amicus, outside of the submitted work. Dr. Bichet reports grants and travel support from and speaker bureau participation for Amicus Therapeutics and Sanofi Genzyme, outside of the submitted work. Dr. Morel reports personal fees from Takeda, outside of the submitted work. Dr. Tocoian and Dr. Lan were employees of Takeda at the time of the study. Dr. West reports grants, personal fees, and travel support from Amicus Therapeutics, Idorsia, Protalix, Sanofi Genzyme, and Takeda, outside of the submitted work.
Figures



References
-
- Kirkilionis AJ, Riddell DC, Spence MW, Fenwick RG. Fabry disease in a large Nova Scotia kindred: carrier detection using leucocyte alpha-galactosidase activity and an Ncol polymorphism detected by an alpha-galactosidase cDNA clone. J Med Genet. 1991;28:232–240. doi: 10.1136/jmg.28.4.232. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

