HIV-1 requires capsid remodelling at the nuclear pore for nuclear entry and integration
- PMID: 34543344
- PMCID: PMC8483370
- DOI: 10.1371/journal.ppat.1009484
HIV-1 requires capsid remodelling at the nuclear pore for nuclear entry and integration
Abstract
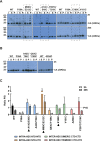
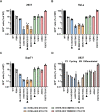
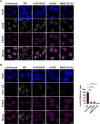
The capsid (CA) lattice of the HIV-1 core plays a key role during infection. From the moment the core is released into the cytoplasm, it interacts with a range of cellular factors that, ultimately, direct the pre-integration complex to the integration site. For integration to occur, the CA lattice must disassemble. Early uncoating or a failure to do so has detrimental effects on virus infectivity, indicating that an optimal stability of the viral core is crucial for infection. Here, we introduced cysteine residues into HIV-1 CA in order to induce disulphide bond formation and engineer hyper-stable mutants that are slower or unable to uncoat, and then followed their replication. From a panel of mutants, we identified three with increased capsid stability in cells and found that, whilst the M68C/E212C mutant had a 5-fold reduction in reverse transcription, two mutants, A14C/E45C and E180C, were able to reverse transcribe to approximately WT levels in cycling cells. Moreover, these mutants only had a 5-fold reduction in 2-LTR circle production, suggesting that not only could reverse transcription complete in hyper-stable cores, but that the nascent viral cDNA could enter the nuclear compartment. Furthermore, we observed A14C/E45C mutant capsid in nuclear and chromatin-associated fractions implying that the hyper-stable cores themselves entered the nucleus. Immunofluorescence studies revealed that although the A14C/E45C mutant capsid reached the nuclear pore with the same kinetics as wild type capsid, it was then retained at the pore in association with Nup153. Crucially, infection with the hyper-stable mutants did not promote CPSF6 re-localisation to nuclear speckles, despite the mutant capsids being competent for CPSF6 binding. These observations suggest that hyper-stable cores are not able to uncoat, or remodel, enough to pass through or dissociate from the nuclear pore and integrate successfully. This, is turn, highlights the importance of capsid lattice flexibility for nuclear entry. In conclusion, we hypothesise that during a productive infection, a capsid remodelling step takes place at the nuclear pore that releases the core complex from Nup153, and relays it to CPSF6, which then localises it to chromatin ready for integration.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

