Latent Class Analysis Reveals COVID-19-related Acute Respiratory Distress Syndrome Subgroups with Differential Responses to Corticosteroids
- PMID: 34543591
- PMCID: PMC8786071
- DOI: 10.1164/rccm.202105-1302OC
Latent Class Analysis Reveals COVID-19-related Acute Respiratory Distress Syndrome Subgroups with Differential Responses to Corticosteroids
Abstract
Rationale: Two distinct subphenotypes have been identified in acute respiratory distress syndrome (ARDS), but the presence of subgroups in ARDS associated with coronavirus disease (COVID-19) is unknown. Objectives: To identify clinically relevant, novel subgroups in COVID-19-related ARDS and compare them with previously described ARDS subphenotypes. Methods: Eligible participants were adults with COVID-19 and ARDS at Columbia University Irving Medical Center. Latent class analysis was used to identify subgroups with baseline clinical, respiratory, and laboratory data serving as partitioning variables. A previously developed machine learning model was used to classify patients as the hypoinflammatory and hyperinflammatory subphenotypes. Baseline characteristics and clinical outcomes were compared between subgroups. Heterogeneity of treatment effect for corticosteroid use in subgroups was tested. Measurements and Main Results: From March 2, 2020, to April 30, 2020, 483 patients with COVID-19-related ARDS met study criteria. A two-class latent class analysis model best fit the population (P = 0.0075). Class 2 (23%) had higher proinflammatory markers, troponin, creatinine, and lactate, lower bicarbonate, and lower blood pressure than class 1 (77%). Ninety-day mortality was higher in class 2 versus class 1 (75% vs. 48%; P < 0.0001). Considerable overlap was observed between these subgroups and ARDS subphenotypes. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RT-PCR cycle threshold was associated with mortality in the hypoinflammatory but not the hyperinflammatory phenotype. Heterogeneity of treatment effect to corticosteroids was observed (P = 0.0295), with improved mortality in the hyperinflammatory phenotype and worse mortality in the hypoinflammatory phenotype, with the caveat that corticosteroid treatment was not randomized. Conclusions: We identified two COVID-19-related ARDS subgroups with differential outcomes, similar to previously described ARDS subphenotypes. SARS-CoV-2 PCR cycle threshold had differential value for predicting mortality in the subphenotypes. The subphenotypes had differential treatment responses to corticosteroids.
Keywords: ARDS; COVID-19; latent class analysis; phenotyping.
Figures

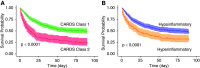


Comment in
-
COVID-19-related Acute Respiratory Distress Syndrome Subphenotypes and Differential Response to Corticosteroids: Time for More Precision?Am J Respir Crit Care Med. 2021 Dec 1;204(11):1241-1243. doi: 10.1164/rccm.202109-2213ED. Am J Respir Crit Care Med. 2021. PMID: 34705609 Free PMC article. No abstract available.
-
Time to Tailor the One-Size-Fits-All Approach?Am J Respir Crit Care Med. 2022 Feb 15;205(4):479-480. doi: 10.1164/rccm.202110-2317LE. Am J Respir Crit Care Med. 2022. PMID: 34818118 Free PMC article. No abstract available.
References
-
- Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA . 2012;307:2526–2533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

