Evaluation of a Digital Handheld Hydrogen Breath Monitor to Diagnose Lactose Malabsorption: Interventional Crossover Study
- PMID: 34544034
- PMCID: PMC8561400
- DOI: 10.2196/33009
Evaluation of a Digital Handheld Hydrogen Breath Monitor to Diagnose Lactose Malabsorption: Interventional Crossover Study
Abstract
Background: Lactose malabsorption is a common condition that affects a broad segment of the population. Clinical diagnosis based on symptom recall can be unreliable and conventional testing can be inconvenient, requiring expensive laboratory-based equipment and conduction of the testing in a clinical setting.
Objective: The aim of this study is to assess the performance of a digital handheld hydrogen breath monitor (GIMate) in diagnosing lactose malabsorption compared to a US Food and Drug Administration (FDA)-cleared device (H2 Check) for the same indication.
Methods: An interventional crossover study was performed in adult participants with a prior confirmed diagnosis of lactose malabsorption or a suspected history of lactose intolerance.
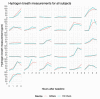
Results: A total of 31 participants (mean age 33.9 years) were enrolled in the study. There was 100% positive percent agreement and 100% negative percent agreement between the GIMate monitor and the H2 Check. Correlation between gastrointestinal symptoms and hydrogen values was positive at 0.82 (P<.001).
Conclusions: The digital handheld GIMate breath monitor achieved equivalent diagnostic performance to that of an FDA-cleared device in the diagnosis of lactose malabsorption.
Trial registration: ClinicalTrials.gov NCT04754724; https://clinicaltrials.gov/ct2/show/NCT04754724.
Keywords: detection; diagnosis; diagnostic; digestion; digestive disease; digital health; evaluation; gastrointestinal; lactose intolerance; medical device; performance; testing.
©Simon C Mathews, Sandy Templeton, Stephanie K Taylor, Sten Harris, Margaret Stewart, Shruti M Raja. Originally published in JMIR Formative Research (https://formative.jmir.org), 18.10.2021.
Conflict of interest statement
Conflicts of Interest: SCM is an officer at Vivante Health with stock options in the company. ST has consulted for Vivante Health and has stock options in the company. SMR, SKT, SH, and MS have no conflicts to declare.
Figures
References
-
- Montalto M, Curigliano V, Santoro L, Vastola M, Cammarota G, Manna R, Gasbarrini A, Gasbarrini G. Management and treatment of lactose malabsorption. World J Gastroenterol. 2006 Jan 14;12(2):187–91. doi: 10.3748/wjg.v12.i2.187. https://www.wjgnet.com/1007-9327/full/v12/i2/187.htm - DOI - PMC - PubMed
-
- Gilat T, Russo S, Gelman-Malachi E, Aldor TA. Lactase in man: a nonadaptable enzyme. Gastroenterology. 1972 Jun;62(6):1125–7. - PubMed
-
- Labayen I, Forga L, González A, Lenoir-Wijnkoop I, Nutr R, Martínez JA. Relationship between lactose digestion, gastrointestinal transit time and symptoms in lactose malabsorbers after dairy consumption. Aliment Pharmacol Ther. 2001 Apr;15(4):543–9. doi: 10.1046/j.1365-2036.2001.00952.x. https://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm:pu... apt952 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical