Safety and Immunogenicity of a Newcastle Disease Virus Vector-Based SARS-CoV-2 Vaccine Candidate, AVX/COVID-12-HEXAPRO (Patria), in Pigs
- PMID: 34544278
- PMCID: PMC8546847
- DOI: 10.1128/mBio.01908-21
Safety and Immunogenicity of a Newcastle Disease Virus Vector-Based SARS-CoV-2 Vaccine Candidate, AVX/COVID-12-HEXAPRO (Patria), in Pigs
Abstract
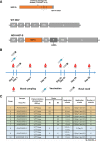
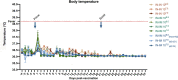
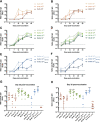
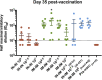
Vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were developed in record time and show excellent efficacy and effectiveness against coronavirus disease 2019 (COVID-19). However, currently approved vaccines cannot meet the global demand. In addition, none of the currently used vaccines is administered intranasally to potentially induce mucosal immunity. Here, we tested the safety and immunogenicity of a second-generation SARS-CoV-2 vaccine that includes a stabilized spike antigen and can be administered intranasally. The vaccine is based on a live Newcastle disease virus vector expressing a SARS-CoV-2 spike protein stabilized in a prefusion conformation with six beneficial proline substitutions (AVX/COVID-12-HEXAPRO; Patria). Immunogenicity testing in the pig model showed that both intranasal and intramuscular application of the vaccine as well as a combination of the two induced strong serum neutralizing antibody responses. Furthermore, substantial reactivity to B.1.1.7, B.1.351, and P.1 spike variants was detected. Finally, no adverse reactions were found in the experimental animals at any dose level or delivery route. These results indicate that the experimental vaccine AVX/COVID-12-HEXAPRO (Patria) is safe and highly immunogenic in the pig model. IMPORTANCE Several highly efficacious vaccines for SARS-CoV-2 have been developed and are used in the population. However, the current production capacity cannot meet the global demand. Therefore, additional vaccines-especially ones that can be produced locally and at low cost-are urgently needed. This work describes preclinical testing of a SARS-CoV-2 vaccine candidate which meets these criteria.
Keywords: COVID-19; HexaPro; NDV; Newcastle disease virus; SARS-CoV-2; coronavirus vaccine; pig model; pigs; prolines; spike; vaccine.
Figures


References
-
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. doi:10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team . 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. doi:10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Pan-American Health Organization. 2021. PAHO Director warns that vaccines alone will not stop current COVID-19 surge. Pan-American Health Organization, Washington, DC. https://www.paho.org/en/news/14-4-2021-paho-director-warns-vaccines-alon....
-
- Sun W, McCroskery S, Liu WC, Leist SR, Liu Y, Albrecht RA, Slamanig S, Oliva J, Amanat F, Schäfer A, Dinnon KH, Innis BL, García-Sastre A, Krammer F, Baric RS, Palese P. 2020. A Newcastle disease virus (NDV) expressing a membrane-anchored spike as a cost-effective inactivated SARS-CoV-2 vaccine. Vaccines (Basel) 8:771. doi:10.3390/vaccines8040771. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
