Choroid plexus APP regulates adult brain proliferation and animal behavior
- PMID: 34544751
- PMCID: PMC8473726
- DOI: 10.26508/lsa.202000703
Choroid plexus APP regulates adult brain proliferation and animal behavior
Abstract
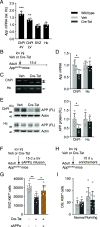
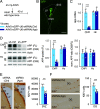
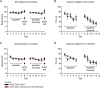
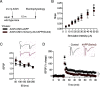
Elevated amyloid precursor protein (APP) expression in the choroid plexus suggests an important role for extracellular APP metabolites such as sAPPα in cerebrospinal fluid. Despite widespread App brain expression, we hypothesized that specifically targeting choroid plexus expression could alter animal physiology. Through various genetic and viral approaches in the adult mouse, we show that choroid plexus APP levels significantly impact proliferation in both subventricular zone and hippocampus dentate gyrus neurogenic niches. Given the role of Aβ peptides in Alzheimer disease pathogenesis, we also tested whether favoring the production of Aβ in choroid plexus could negatively affect niche functions. After AAV5-mediated long-term expression of human mutated APP specifically in the choroid plexus of adult wild-type mice, we observe reduced niche proliferation, reduced hippocampus APP expression, behavioral defects in reversal learning, and deficits in hippocampal long-term potentiation. Our findings highlight the unique role played by the choroid plexus in regulating brain function and suggest that targeting APP in choroid plexus may provide a means to improve hippocampus function and alleviate disease-related burdens.
© 2021 Arnaud et al.
Conflict of interest statement
A Prochiantz is a cofounder of BrainEver SAS; other authors report no biomedical financial interests or potential conflicts of interest.
Figures



References
-
- Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, Berkutzki T, Barnett-Itzhaki Z, Bezalel D, Wyss-Coray T, et al. (2014) Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 346: 89–93. 10.1126/science.1252945 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
