Systematic Analysis of Brain MRI Findings in Adaptor Protein Complex 4-Associated Hereditary Spastic Paraplegia
- PMID: 34544818
- PMCID: PMC8601212
- DOI: 10.1212/WNL.0000000000012836
Systematic Analysis of Brain MRI Findings in Adaptor Protein Complex 4-Associated Hereditary Spastic Paraplegia
Abstract
Background and objectives: AP-4-associated hereditary spastic paraplegia (AP-4-HSP: SPG47, SPG50, SPG51, SPG52) is an emerging cause of childhood-onset hereditary spastic paraplegia and mimic of cerebral palsy. This study aims to define the spectrum of brain MRI findings in AP-4-HSP and to investigate radioclinical correlations.
Methods: We performed a systematic qualitative and quantitative analysis of 107 brain MRI studies from 76 individuals with genetically confirmed AP-4-HSP and correlation with clinical findings including surrogates of disease severity.
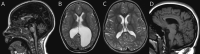
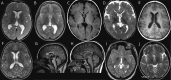
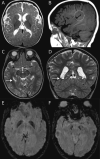
Results: We define AP-4-HSP as a disorder of gray and white matter and demonstrate that abnormal myelination is common and that metrics of reduced white matter volume correlate with severity of motor symptoms. We identify a common diagnostic imaging signature consisting of (1) a thin splenium of the corpus callosum, (2) an absent or thin anterior commissure, (3) characteristic signal abnormalities of the forceps minor ("ears of the grizzly sign"), and (4) periventricular white matter abnormalities. The presence of 2 or more of these findings has a sensitivity of ∼99% for detecting AP-4-HSP; the combination of all 4 is found in ∼45% of cases. Compared to other HSPs with a thin corpus callosum, the absent anterior commissure appears to be specific to AP-4-HSP. Our analysis identified a subset of patients with polymicrogyria, underscoring the role of AP-4 in early brain development. These patients displayed a higher prevalence of seizures and status epilepticus, many at a young age.
Discussion: Our findings define the MRI spectrum of AP-4-HSP, providing opportunities for early diagnosis, identification of individuals at risk for complications, and a window into the role of the AP-4 complex in brain development and neurodegeneration.
© 2021 American Academy of Neurology.
Figures

References
-
- Shribman S, Reid E, Crosby AH, Houlden H, Warner TT. Hereditary spastic paraplegia: from diagnosis to emerging therapeutic approaches. Lancet Neurol. 2019;18(12):1136-1146. - PubMed
-
- Erichsen AK, Koht J, Stray-Pedersen A, Abdelnoor M, Tallaksen CM. Prevalence of hereditary ataxia and spastic paraplegia in southeast Norway: a population-based study. Brain. 2009;132(Pt 6):1577-1588. - PubMed
-
- Ebrahimi-Fakhari D, Behne R, Davies AK, Hirst J. AP-4-associated hereditary spastic paraplegia. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. University of Washington; 2018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
