Extracellular matrix protein N-glycosylation mediates immune self-tolerance in Drosophila melanogaster
- PMID: 34544850
- PMCID: PMC8488588
- DOI: 10.1073/pnas.2017460118
Extracellular matrix protein N-glycosylation mediates immune self-tolerance in Drosophila melanogaster
Abstract
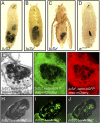
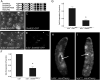
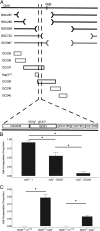
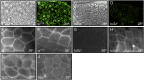
In order to respond to infection, hosts must distinguish pathogens from their own tissues. This allows for the precise targeting of immune responses against pathogens and also ensures self-tolerance, the ability of the host to protect self tissues from immune damage. One way to maintain self-tolerance is to evolve a self signal and suppress any immune response directed at tissues that carry this signal. Here, we characterize the Drosophila tuSz1 mutant strain, which mounts an aberrant immune response against its own fat body. We demonstrate that this autoimmunity is the result of two mutations: 1) a mutation in the GCS1 gene that disrupts N-glycosylation of extracellular matrix proteins covering the fat body, and 2) a mutation in the Drosophila Janus Kinase ortholog that causes precocious activation of hemocytes. Our data indicate that N-glycans attached to extracellular matrix proteins serve as a self signal and that activated hemocytes attack tissues lacking this signal. The simplicity of this invertebrate self-recognition system and the ubiquity of its constituent parts suggests it may have functional homologs across animals.
Keywords: autoimmunity; innate immunity; protein N-glycosylation; self-recognition; self-tolerance.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Danilova N., The evolution of immune mechanisms. J. Exp. Zoolog. B Mol. Dev. Evol. 306, 496–520 (2006). - PubMed
-
- Janeway C. A. Jr, Medzhitov R., Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002). - PubMed
-
- Medzhitov R., Janeway C. A. Jr, Decoding the patterns of self and nonself by the innate immune system. Science 296, 298–300 (2002). - PubMed
-
- Cooper M. D., Alder M. N., The evolution of adaptive immune systems. Cell 124, 815–822 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

