Norrie disease protein is essential for cochlear hair cell maturation
- PMID: 34544869
- PMCID: PMC8488680
- DOI: 10.1073/pnas.2106369118
Norrie disease protein is essential for cochlear hair cell maturation
Abstract
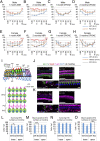
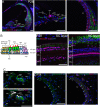
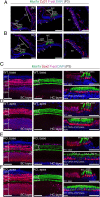
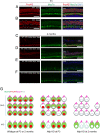
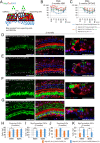
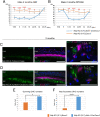
Mutations in the gene for Norrie disease protein (Ndp) cause syndromic deafness and blindness. We show here that cochlear function in an Ndp knockout mouse deteriorated with age: At P3-P4, hair cells (HCs) showed progressive loss of Pou4f3 and Gfi1, key transcription factors for HC maturation, and Myo7a, a specialized myosin required for normal function of HC stereocilia. Loss of expression of these genes correlated to increasing HC loss and profound hearing loss by 2 mo. We show that overexpression of the Ndp gene in neonatal supporting cells or, remarkably, up-regulation of canonical Wnt signaling in HCs rescued HCs and cochlear function. We conclude that Ndp secreted from supporting cells orchestrates a transcriptional network for the maintenance and survival of HCs and that increasing the level of β-catenin, the intracellular effector of Wnt signaling, is sufficient to replace the functional requirement for Ndp in the cochlea.
Keywords: Norrie disease; Wnt signaling; cochlear hair cells.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Halpin C., Sims K., Twenty years of audiology in a patient with Norrie disease. Int. J. Pediatr. Otorhinolaryngol. 72, 1705–1710 (2008). - PubMed
-
- Luhmann U. F., et al. ., Role of the Norrie disease pseudoglioma gene in sprouting angiogenesis during development of the retinal vasculature. Invest. Ophthalmol. Vis. Sci. 46, 3372–3382 (2005). - PubMed
-
- Jin T., George Fantus I., Sun J., Wnt and beyond Wnt: Multiple mechanisms control the transcriptional property of beta-catenin. Cell. Signal. 20, 1697–1704 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

