The relationship of polluted air and drinking water sources with the prevalence of systemic lupus erythematosus: a provincial population-based study
- PMID: 34545152
- PMCID: PMC8452734
- DOI: 10.1038/s41598-021-98111-8
The relationship of polluted air and drinking water sources with the prevalence of systemic lupus erythematosus: a provincial population-based study
Abstract
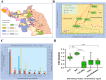
Environmental exposures interact with genetic factors has been thought to influence susceptibility of systemic lupus erythematosus (SLE) development. To evaluate the effects of environmental exposures on SLE, we conducted a population-based cohort study across Jiangsu Province, China, to examine the associations between the living environment including air and water pollution, population density, economic income level, etc. and the prevalence and mortality of hospitalized SLE (h-SLE) patients. A total of 2231 h-SLE patients were retrieved from a longitudinal SLE database collected by the Jiangsu Lupus Collaborative Group from 1999 to 2009. The results showed that: It existed regional differences on the prevalence of h-SLE patients in 96 administrative districts; The distribution of NO2 air concentration monitored by atmospheric remote sensors showed that three of the ultra-high-prevalence districts were located in the concentrated chemical industry emission area; h-SLE patient prevalence was positively correlated with the excessive levels of nitrogen in drinking water; The positive ratio of pericarditis and proteinuria was positively correlated with the prevalence of h-SLE patients and pollution not only induced a high h-SLE patient prevalence but also a higher mortality rate, which might be attributed to NOx pollution in the air and drinking water. In summary, our data suggested that NOx in air and drinking water may be one of the important predispositions of SLE, especially for patients with renal involvement.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Justiz Vaillant, A. A., Goyal, A., Bansal, P. & Varacallo, M. In StatPearls (2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

