Predictors of mortality in thrombotic thrombocytopenia after adenoviral COVID-19 vaccination: the FAPIC score
- PMID: 34545400
- PMCID: PMC8500026
- DOI: 10.1093/eurheartj/ehab592
Predictors of mortality in thrombotic thrombocytopenia after adenoviral COVID-19 vaccination: the FAPIC score
Abstract
Aims: The clinical manifestation and outcomes of thrombosis with thrombocytopenia syndrome (TTS) after adenoviral COVID-19 vaccine administration are largely unknown due to the rare nature of the disease. We aimed to analyse the clinical presentation, treatment modalities, outcomes, and prognostic factors of adenoviral TTS, as well as identify predictors for mortality.
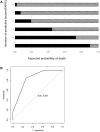
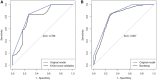
Methods and results: PubMed, Scopus, Embase, and Web of Science databases were searched and the resulting articles were reviewed. A total of 6 case series and 13 case reports (64 patients) of TTS after ChAdOx1 nCoV-19 vaccination were included. We performed a pooled analysis and developed a novel scoring system to predict mortality. The overall mortality of TTS after ChAdOx1 nCoV-19 vaccination was 35.9% (23/64). In our analysis, age ≤60 years, platelet count <25 × 103/µL, fibrinogen <150 mg/dL, the presence of intracerebral haemorrhage (ICH), and the presence of cerebral venous thrombosis (CVT) were significantly associated with death and were selected as predictors for mortality (1 point each). We named this novel scoring system FAPIC (fibrinogen, age, platelet count, ICH, and CVT), and the C-statistic for the FAPIC score was 0.837 (95% CI 0.732-0.942). Expected mortality increased with each point increase in the FAPIC score, at 2.08, 6.66, 19.31, 44.54, 72.94, and 90.05% with FAPIC scores 0, 1, 2, 3, 4, and 5, respectively. The FAPIC scoring model was internally validated through cross-validation and bootstrapping, then externally validated on a panel of TTS patients after Ad26.COV2.S administration.
Conclusions: Fibrinogen levels, age, platelet count, and the presence of ICH and CVT were significantly associated with mortality in patients with TTS, and the FAPIC score comprising these risk factors could predict mortality. The FAPIC score could be used in the clinical setting to recognize TTS patients at high risk of adverse outcomes and provide early intensive interventions including intravenous immunoglobulins and non-heparin anticoagulants.
Keywords: COVID-19 vaccine; Cerebral venous thrombosis; ChAdOx1 nCoV-19; Thrombotic thrombocytopenia syndrome; Vaccine-induced thrombotic thrombocytopenia.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
Thromboinflammatory findings and clinical predictors of mortality in vaccine-induced immune thrombotic thrombocytopenia.Eur Heart J. 2021 Oct 14;42(39):4073-4076. doi: 10.1093/eurheartj/ehab585. Eur Heart J. 2021. PMID: 34545405 No abstract available.
References
-
- Johns H. Coronavirus Resource Center. COVID-19 Map [Internet]. 2021. https://coronavirus.jhu.edu/map.html (28 April 2021).
-
- Gavi C. COVID-19 Vaccine Advance Market Commitment [Internet]. 2021. https://www.gavi.org/gavi-covax-amc (28 April 2021).
-
- European Medicines Agency. COVID-19 vaccines: authorised [Internet]. 2021. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-thr... (29 April 2021).
-
- Food and Drug Administration. COVID-19 Vaccines. FDA [Internet]. 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise... (29 April 2021).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

