Three-Year Results from the Venovo Venous Stent Study for the Treatment of Iliac and Femoral Vein Obstruction
- PMID: 34545448
- PMCID: PMC8451739
- DOI: 10.1007/s00270-021-02975-2
Three-Year Results from the Venovo Venous Stent Study for the Treatment of Iliac and Femoral Vein Obstruction
Erratum in
-
Correction to: Three-Year Results from the Venovo Venous Stent Study for the Treatment of Iliac and Femoral Vein Obstruction.Cardiovasc Intervent Radiol. 2021 Dec;44(12):2027. doi: 10.1007/s00270-021-02982-3. Cardiovasc Intervent Radiol. 2021. PMID: 34668057 Free PMC article. No abstract available.
Abstract
Purpose: To assess safety and patency of the Venovo venous stent for the treatment of iliofemoral vein obstruction.
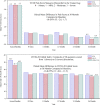
Materials and methods: Twenty-two international centers enrolled 170 patients in the VERNACULAR study (93 post-thrombotic syndrome; 77 non-thrombotic iliac vein lesions). Primary outcome measures were major adverse events at 30 days and 12-month primary patency (freedom from target vessel revascularization, thrombotic occlusion, or stenosis > 50%). Secondary outcomes included the Venous Clinical Severity Score Pain Assessment and Chronic Venous Quality-of-Life Questionnaire assessments (hypothesis tested). Secondary observations included primary patency, target vessel and lesion revascularization (TVR/TLR), and assessment of stent integrity through 36 months.
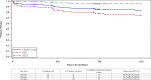
Results: Freedom from major adverse events through 30 days was 93.5%, statistically higher than a pre-specified performance goal of 89% (p = 0.032) while primary patency at 12 months was 88.6%, also statistically higher than a performance goal of 74% (p < 0.0001). Mean quality-of-life measures were statistically improved compared to baseline values at 12 months (p < 0.0001). Primary patency at 36 months was 84% (Kaplan-Meier analysis) while freedom from TVR/TLR was 88.1%. There was no stent embolization/migration, and no core laboratory assessed stent fractures reported through 36 months. Six deaths were reported; none adjudicated as device or procedure related.
Conclusion: The Venovo venous stent was successfully deployed in obstructive iliofemoral vein lesions and met the pre-specified primary outcome measures through 12 months. At 3 years, primary patency was 84%, reintervention rates were low, standardized quality-of-life and pain measures improved from baseline, and there was no stent migration or fractures.
Level of evidence: Level 2-prospective, multicenter, controlled clinical study without a concurrent control or randomization. Pre-specified endpoints were hypothesis-tested to performance goals derived from peer-reviewed clinical literature. REGISTRATION CLINICALTRIALS.GOV: Unique Identifier NCT02655887.
Keywords: Iliac and femoral vein occlusive disease; Iliofemoral venous obstruction; Non-thrombotic iliac vein lesion; Percutaneous endovenous stent; Post-thrombotic syndrome.
© 2021. The Author(s).
Conflict of interest statement
MDD is a member of the Scientific Advisory Board of W.L. Gore and consultant to Cook Medical. GO is a consultant and received speaker honoraria from Cook Medical, C. R. Bard (Bard)/Becton, Dickinson and Company (BD), Boston Scientific, Philips, Medtronic, WhiteSwell, VeinWay, and Vivasure as well as a shareholder in Marvao Medical. NWS has received research and educational grants from Bard/BD, Boston Scientific, Phillips, AngioDynamics, and VentureMed Group, is on the advisory board of VentureMed group and CSI, and has received speaker honoraria from Janssen, Boehringer Ingelheim, Lilly, Kiniksa, Amgen, and Esperion. ML has received research grants and speaker honoraria from Bard/BD. BPM has received research and educational grants from Boston Scientific, Getinge, and Medtronic Australia as well as speaker honoraria from Bard/BD, Boston Scientific, Getinge, Gore, and Medtronic Australia. RAS is an employee and shareholder of Becton, Dickinson and Company.
Figures
References
-
- Enden T, Haig Y, Klow NE, Slagsvold CE, Sandvik L, Ghanima W, Hafsahl G, Holme PA, Holmen LO, Njaastad AM, Sandbaek G, Sandset PM, CaVenT Study Group Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomized controlled trial. Lancet. 2012;379:31–38. doi: 10.1016/S0140-6736(11)61753-4. - DOI - PubMed
-
- Haig Y, Enden T, Grotta O, Klow NE, Slagsvold CE, Ghanima W, Sandvik L, Hafsahl G, Holme PA, Holmen LO, Njaastad AM, Sandbaek G, Sandset PM, CaVenT Study Group Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label randomized controlled trial. Lancet Haematol. 2016;3:e64–e71. doi: 10.1016/S2352-3026(15)00248-3. - DOI - PubMed
-
- Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, Magnuson E, Razavi MK, Comerota AJ, Gornik HL, Murphy TP, Lewis L, Duncan JR, Nieters P, Derfler MC, Filion M, Gu CS, Kee S, Schnieder J, Saad N, Blander M, Moll S, Sacks D, Lin J, Rundback J, Garcia M, Razdan R, Vander-Woude E, Marques V, Kearon C, ATTRACT Trial Investigators Pharmacomechanical catheter-directed thrombolysis for deep vein thrombosis. N Eng J Med. 2017;377:2240–2252. doi: 10.1056/NEJMoa1615066. - DOI - PMC - PubMed
-
- Gutzeit A, Zollikofer CHL, Dettling-Pizzolato M, Graf N, Largiade`r J, Binkert CA. Endovascular stent treatment for symptomatic benign iliofemoral venous occlusive disease: Long-term results 1987–2009. Cardiovasc Intervent Radiol. 2011;34:542–549. doi: 10.1007/s00270-010-9927-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

