Biosensor-Enabled Multiplexed On-Site Therapeutic Drug Monitoring of Antibiotics
- PMID: 34545651
- PMCID: PMC11468941
- DOI: 10.1002/adma.202104555
Biosensor-Enabled Multiplexed On-Site Therapeutic Drug Monitoring of Antibiotics
Abstract
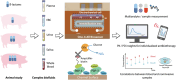
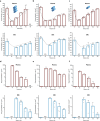
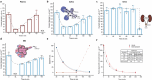
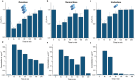
Personalized antibiotherapy ensures that the antibiotic concentration remains in the optimal therapeutic window to maximize efficacy, minimize side effects, and avoid the emergence of drug resistance due to insufficient dosing. However, such individualized schemes need frequent sampling to tailor the blood antibiotic concentrations. To optimally integrate therapeutic drug monitoring (TDM) into the clinical workflow, antibiotic levels can either be measured in blood using point-of-care testing (POCT), or can rely on noninvasive sampling. Here, a versatile biosensor with an antibody-free assay for on-site TDM is presented. The platform is evaluated with an animal study, where antibiotic concentrations are quantified in different matrices including whole blood, plasma, urine, saliva, and exhaled breath condensate (EBC). The clearance and the temporal evaluation of antibiotic levels in EBC and plasma are demonstrated. Influence of matrix effects on measured drug concentrations is determined by comparing the plasma levels with those in noninvasive samples. The system's potential for blood-based POCT is further illustrated by tracking ß-lactam concentrations in untreated blood samples. Finally, multiplexing capabilities are explored successfully for multianalyte/sample analysis. By enabling a rapid, low-cost, sample-independent, and multiplexed on-site TDM, this system can shift the paradigm of "one-size-fits-all" strategy.
Keywords: exhaled breath condensate; multiplexing; noninvasive diagnostics; point-of-care testing; ß-lactam antibiotics.
© 2021 The Authors. Advanced Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ates H. C., Roberts J. A., Lipman J., Cass A. E. G., Urban G. A., Dincer C., Trends Biotechnol. 2020, 38, 1262. - PubMed
-
- Roberts J. A., Ulldemolins M., Roberts M. S., McWhinney B., Ungerer J., Paterson D. L., Lipman J., Int. J. Antimicrob. Agents 2010, 36, 332. - PubMed
-
- Wong G., Briscoe S., McWhinney B., Ally M., Ungerer J., Lipman J., Roberts J. A., J. Antimicrob. Chemother. 2018, 73, 3087. - PubMed
-
- Jelliffe R., Neely M., Bayard D., Handb. Anal. Sep. 2020, 7, 135.
-
- Individualized Drug Therapy for Patients (Eds: Jelliffe R. W., Neely M.), Elsevier B.V., The Netherlands 2017.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

