DARPP-32 distinguishes a subset of adult-born neurons in zebra finch HVC
- PMID: 34545948
- PMCID: PMC9495268
- DOI: 10.1002/cne.25245
DARPP-32 distinguishes a subset of adult-born neurons in zebra finch HVC
Abstract
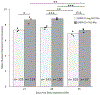
Adult male zebra finches (Taeniopygia guttata) continually incorporate adult-born neurons into HVC, a telencephalic brain region necessary for the production of learned song. These neurons express activity-dependent immediate early genes (e.g., zenk and c-fos) following song production, suggesting that these neurons are active during song production. Half of these adult-born HVC neurons (HVC NNs) can be backfilled from the robust nucleus of the arcopallium (RA) and are a part of the vocal motor pathway underlying learned song production, but the other half do not backfill from RA, and they remain to be characterized. Here, we used cell birth-dating, retrograde tract tracing, and immunofluorescence to demonstrate that half of all HVC NNs express the phosphoprotein DARPP-32, a protein associated with dopamine receptor expression. We also demonstrate that DARPP-32+ HVC NNs are contacted by tyrosine hydroxylase immunoreactive fibers, suggesting that they receive catecholaminergic input, have transiently larger nuclei than DARPP-32-neg HVC NNs, and do not backfill from RA. Taken together, these findings help characterize a group of HVC NNs that have no apparent projections to RA and so far have eluded positive identification other than HVC NN status.
Keywords: HVC; dopamine; neurogenesis; song; zebra finch.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTERESTS
The authors declare no competing financial interests.
Figures

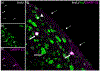
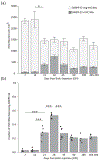

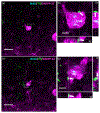
References
-
- Appeltants D, Absil P, Balthazart J, & Ball GF (2000). Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. Journal of Chemical Neuroanatomy, 18, 117–133 - PubMed
-
- Appeltants D, Ball GF, & Balthazart J (2001). The distribution of tyrosine hydroxylase in the canary brain: Demonstration of a specific and sexually dimorphic catecholaminergic innervation of the telencephalic song control nuclei. Cell and Tissue Research, 304, 237–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

