Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs Tricuspid Aortic Stenosis and Mortality or Stroke Among Patients at Low Surgical Risk
- PMID: 34546301
- PMCID: PMC8456392
- DOI: 10.1001/jama.2021.13346
Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs Tricuspid Aortic Stenosis and Mortality or Stroke Among Patients at Low Surgical Risk
Abstract
Importance: There are limited data on outcomes of transcatheter aortic valve replacement (TAVR) for bicuspid aortic stenosis in patients at low surgical risk.
Objective: To compare the outcomes of TAVR with a balloon-expandable valve for bicuspid vs tricuspid aortic stenosis in patients who are at low surgical risk.
Design, setting, and participants: Registry-based cohort study of patients undergoing TAVR at 684 US centers. Participants were enrolled in the Society of Thoracic Surgeons (STS)/American College of Cardiology Transcatheter Valve Therapies Registry from June 2015 to October 2020. Among 159 661 patients (7058 bicuspid, 152 603 tricuspid), 37 660 patients (3243 bicuspid and 34 417 tricuspid) who were at low surgical risk (defined as STS risk score <3%) were included in the analysis.
Exposures: TAVR for bicuspid vs tricuspid aortic stenosis.
Main outcomes and measures: Coprimary outcomes were 30-day and 1-year mortality and stroke. Secondary outcomes included procedural complications and valve hemodynamics.
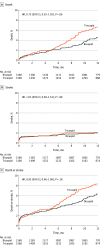
Results: Among 159 661 patients (7058 bicuspid; 152 603 tricuspid), 3168 propensity-matched pairs of patients with bicuspid and tricuspid aortic stenosis at low surgical risk were analyzed (mean age, 69 years; 69.8% men; mean [SD] STS-predicted risk of mortality, 1.7% [0.6%] for bicuspid and 1.7% [0.7%] for tricuspid). There was no significant difference between the bicuspid and tricuspid groups' rates of death at 30 days (0.9% vs 0.8%; hazard ratio [HR], 1.18 [95% CI, 0.68-2.03]; P = .55) and at 1 year (4.6% vs 6.6%; HR, 0.75 [95% CI, 0.55-1.02]; P = .06) or stroke at 30 days (1.4% vs 1.2%; HR, 1.14 [95% CI, 0.73-1.78]; P = .55) and at 1 year (2.0% vs 2.1%; HR 1.03 [95% CI, 0.69-1.53]; P = .89).There were no significant differences between the bicuspid and tricuspid groups in procedural complications, valve hemodynamics (aortic valve gradient: 13.2 mm Hg vs 13.5 mm Hg; absolute risk difference [RD], 0.3 mm Hg [95% CI, -0.9 to 0.3 mm Hg]), and moderate or severe paravalvular leak (3.4% vs 2.1%; absolute RD, 1.3% [95% CI, -0.6% to 3.2%]).
Conclusions and relevance: In this preliminary, registry-based study of propensity-matched patients at low surgical risk who had undergone TAVR for aortic stenosis, patients treated for bicuspid vs tricuspid aortic stenosis had no significant difference in mortality or stroke at 30 days or 1 year. Because of the potential for selection bias and absence of a control group treated surgically for bicuspid aortic stenosis, randomized trials are needed to adequately assess the efficacy and safety of transcatheter aortic valve replacement for bicuspid aortic stenosis in patients at low surgical risk.
Conflict of interest statement
Figures
Comment in
-
Transcatheter Valve Replacement for Bicuspid Aortic Stenosis.JAMA. 2021 Sep 21;326(11):1009-1010. doi: 10.1001/jama.2021.13229. JAMA. 2021. PMID: 34546317 No abstract available.
-
The Puzzle of TAVR for Bicuspid AS: Still Missing a Piece?J Cardiothorac Vasc Anesth. 2022 May;36(5):1225-1227. doi: 10.1053/j.jvca.2021.12.031. Epub 2022 Jan 3. J Cardiothorac Vasc Anesth. 2022. PMID: 35131165 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

