CD40 Expressed in Endothelial Cells Promotes Upregulation of ICAM-1 But Not Pro-Inflammatory Cytokines, NOS2 and P2X7 in the Diabetic Retina
- PMID: 34546322
- PMCID: PMC8458989
- DOI: 10.1167/iovs.62.12.22
CD40 Expressed in Endothelial Cells Promotes Upregulation of ICAM-1 But Not Pro-Inflammatory Cytokines, NOS2 and P2X7 in the Diabetic Retina
Abstract
Purpose: CD40 is an upstream inducer of inflammation in the diabetic retina. CD40 is upregulated in retinal endothelial cells in diabetes. The purpose of this study was to determine whether expression of CD40 in endothelial cells is sufficient to promote inflammatory responses in the retina of diabetic mice.
Methods: Transgenic mice with CD40 expression restricted to endothelial cells (Trg-CD40 EC), transgenic control mice (Trg-Ctr), B6, and CD40-/- mice were made diabetic using streptozotocin. Leukostasis was assessed using FITC-conjugated ConA. Pro-inflammatory molecule expression was examined by real-time PCR, immunohistochemistry, ELISA, or flow cytometry. Release of ATP was assessed by ATP bioluminescence.
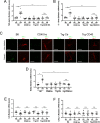
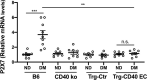
Results: Diabetic B6 and Trg-CD40 EC mice exhibited increased retinal mRNA levels of ICAM-1, higher ICAM-1 expression in endothelial cells, and increased leukostasis. These responses were not detected in diabetic mice that lacked CD40 (CD40-/- and Trg-Ctr). Diabetic B6 but not Trg-CD40 EC mice upregulated TNF-α, IL-1β, and NOS2 mRNA levels. CD40 stimulation in retinal endothelial cells upregulated ICAM-1 but not TNF-α, IL-1β, or NOS2. CD40 ligation did not trigger ATP release by retinal endothelial cells or pro-inflammatory cytokine production in bystander myeloid cells. In contrast to diabetic B6 mice, diabetic Trg-CD40 EC mice did not upregulate P2X7 mRNA levels in the retina.
Conclusions: Endothelial cell CD40 promotes ICAM-1 upregulation and leukostasis. In contrast, endothelial cell CD40 does not lead to pro-inflammatory cytokine and NOS2 upregulation likely because it does not activate purinergic-mediated pro-inflammatory molecule expression by myeloid cells or induce expression of these pro-inflammatory molecules in endothelial cells.
Conflict of interest statement
Disclosure:
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

