Maternal vs Fetal Origin of Placental Intervillous Thrombi
- PMID: 34546332
- PMCID: PMC8500002
- DOI: 10.1093/ajcp/aqab139
Maternal vs Fetal Origin of Placental Intervillous Thrombi
Abstract
Objectives: To determine maternal vs fetal origin for blood in placental intervillous thrombi (IVTs).
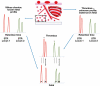
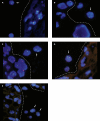
Methods: We used comparative analysis of microsatellites (short tandem repeats [STRs]), sex chromosome fluorescence in situ hybridization (FISH), and immunohistochemistry (IHC) for fetal (ɑ-fetoprotein [AFP]) and maternal (immunoglobulin M [IgM]) serum proteins to distinguish the origin of IVTs. Using an informatics approach, we tested the association between IVTs and fetomaternal hemorrhage (FMH).
Results: In 9 of 10 cases, the preponderance of evidence showed that the thrombus was mostly or entirely maternal in origin. In 1 case, the thrombus was of mixed origins. STR testing was prone to contamination by entrapped fetal villi. FISH was useful but limited only to cases with male fetuses. IgM showed stronger staining than AFP in 9 cases, supporting maternal origin. By informatics, we found no association between IVTs and FMH.
Conclusions: Evidence supports a maternal origin for blood in IVTs. IHC for IgM and AFP may be clinically useful in determining maternal vs fetal contribution to IVTs.
Keywords: COVID-19; FISH; Immunohistochemistry; Perinatal; Placenta; STR; Thrombi.
© American Society for Clinical Pathology, 2021. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Baergen RN. Manual of Pathology of the Human Placenta. 2nd ed. New York: Springer; 2011.
-
- Kaplan C, Blanc WA, Elias J. Identification of erythrocytes in intervillous thrombi: a study using immunoperoxidase identification of hemoglobins. Hum Pathol. 1982;13:554-557. - PubMed
-
- Basnet KM, Bentley-Lewis R, Wexler DJ, et al. . Prevalence of intervillous thrombi is increased in placentas from pregnancies complicated by diabetes. Pediatr Dev Pathol. 2016;19:502-505. - PubMed
-
- Ravishankar S, Migliori A, Struminsky J, et al. . Placental findings in feto-maternal hemorrhage in livebirth and stillbirth. Pathol Res Pract. 2017;213:301-304. - PubMed
-
- Gille J, Schwerd W, von Criegern T. Composition of intervillous thrombi from maternal and fetal blood. Z Geburtshilfe Perinatol. 1986;190:133-136. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

