Implication of folate deficiency in CYP2U1 loss of function
- PMID: 34546337
- PMCID: PMC8480666
- DOI: 10.1084/jem.20210846
Implication of folate deficiency in CYP2U1 loss of function
Abstract
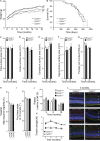
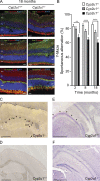
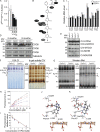
Hereditary spastic paraplegias are heterogeneous neurodegenerative disorders. Understanding of their pathogenic mechanisms remains sparse, and therapeutic options are lacking. We characterized a mouse model lacking the Cyp2u1 gene, loss of which is known to be involved in a complex form of these diseases in humans. We showed that this model partially recapitulated the clinical and biochemical phenotypes of patients. Using electron microscopy, lipidomic, and proteomic studies, we identified vitamin B2 as a substrate of the CYP2U1 enzyme, as well as coenzyme Q, neopterin, and IFN-α levels as putative biomarkers in mice and fluids obtained from the largest series of CYP2U1-mutated patients reported so far. We also confirmed brain calcifications as a potential biomarker in patients. Our results suggest that CYP2U1 deficiency disrupts mitochondrial function and impacts proper neurodevelopment, which could be prevented by folate supplementation in our mouse model, followed by a neurodegenerative process altering multiple neuronal and extraneuronal tissues.
© 2021 Pujol et al.
Conflict of interest statement
Disclosures: F. Darios reports "other" from Dynacure SAS outside the submitted work. D. Galatolo is supported by a grant from Treat SPG56. G. Stevanin reports grants from ASL-HSP association, the Tom-Wahlig-Stiftung Foundation, and the European Union 7th Framework Programme (Neuromics); non-financial support from Agence Nationale de la Recherche (framework programme Investissements d'Avenir) during the conduct of the study; and grants from Biogen outside the submitted work. N. Vilain reports grants from Fondation Bettencourt-Schueller, Fondation Servier, Union Nationale pour les Intérêts de la Médecine, and Fondation pour la Recherche sur l'Alzheimer; non-financial support from Movement Disorders Society, Merz-Pharma, and GE Healthcare SAS; and "other" from Biogen, Eisai, Eli-Lilly, Roche, Janssen - Johnson & Johnson, and Alector outside the submitted work. No other disclosures were reported.
Figures

References
-
- Bibi, F., Efthymiou S., Bourinaris T., Tariq A., Zafar F., Rana N., Salpietro V., Houlden H., Raja G.K., Saeed S., and Minhas N.M.. SYNaPS Study Group . 2020. Rare novel CYP2U1 and ZFYVE26 variants identified in two Pakistani families with spastic paraplegia. J. Neurol. Sci. 411:116669. 10.1016/j.jns.2020.116669 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

