The brain-derived neurotrophic factor prompts platelet aggregation and secretion
- PMID: 34546355
- PMCID: PMC8945584
- DOI: 10.1182/bloodadvances.2020004098
The brain-derived neurotrophic factor prompts platelet aggregation and secretion
Abstract
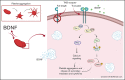
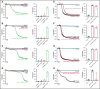
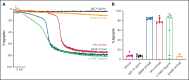
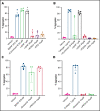
Brain-derived neurotrophic factor (BDNF) has both autocrine and paracrine roles in neurons, and its release and signaling mechanisms have been extensively studied in the central nervous system. Large quantities of BDNF have been reported in circulation, essentially stored in platelets with concentrations reaching 100- to 1000-fold those of neurons. Despite this abundance, the function of BDNF in platelet biology has not been explored. At low concentrations, BDNF primed platelets, acting synergistically with classical agonists. At high concentrations, BDNF induced complete biphasic platelet aggregation that in part relied on amplification from secondary mediators. Neurotrophin-4, but not nerve growth factor, and an activating antibody against the canonical BDNF receptor tropomyosin-related kinase B (TrkB) induced similar platelet responses to BDNF, suggesting TrkB could be the mediator. Platelets expressed, both at their surface and in their intracellular compartment, a truncated form of TrkB lacking its tyrosine kinase domain. BDNF-induced platelet aggregation was prevented by inhibitors of Ras-related C3 botulinum toxin substrate 1 (Rac1), protein kinase C, and phosphoinositide 3-kinase. BDNF-stimulated platelets secreted a panel of angiogenic and inflammatory cytokines, which may play a role in maintaining vascular homeostasis. Two families with autism spectrum disorder were found to carry rare missense variants in the BDNF gene. Platelet studies revealed defects in platelet aggregation to low concentrations of collagen, as well as reduced adenosine triphosphate secretion in response to adenosine diphosphate. In summary, circulating BDNF levels appear to regulate platelet activation, aggregation, and secretion through activation of a truncated TrkB receptor and downstream kinase-dependent signaling.
© 2021 by The American Society of Hematology.
Figures




References
-
- Lu B, Pang PT, Woo NH.. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6(8):603-614. - PubMed
-
- Klein R, Conway D, Parada LF, Barbacid M.. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990;61(4):647-656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

