Metronomic chemotherapy (mCHT) in metastatic triple-negative breast cancer (TNBC) patients: results of the VICTOR-6 study
- PMID: 34546500
- PMCID: PMC8558172
- DOI: 10.1007/s10549-021-06375-5
Metronomic chemotherapy (mCHT) in metastatic triple-negative breast cancer (TNBC) patients: results of the VICTOR-6 study
Abstract
Purpose: Triple-negative breast cancer (TNBC) represents a subtype of breast cancer which lacks the expression of oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2): TNBC accounts for approximately 20% of newly diagnosed breast cancers and is associated with younger age at diagnosis, greater recurrence risk and shorter survival time. Therapeutic options are very scarce. Aim of the present analysis is to provide further insights into the clinical activity of metronomic chemotherapy (mCHT), in a real-life setting.
Methods: We used data included in the VICTOR-6 study for the present analysis. VICTOR-6 is an Italian multicentre retrospective cohort study, which collected data of metastatic breast cancer (MBC) patients who have received mCHT between 2011 and 2016. Amongst the 584 patients included in the study, 97 were triple negative. In 40.2% of the TNBC patients, mCHT was the first chemotherapy treatment, whereas 32.9% had received 2 or more lines of treatment for the metastatic disease. 45.4% out of 97 TNBC patients received a vinorelbine (VRL)-based regimen, which resulted in the most used type of mCHT, followed by cyclophosphamide (CTX)-based regimens (30.9%) and capecitabine (CAPE)-based combinations (22.7%).
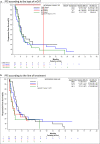
Results: Overall response rate (ORR) and disease control rate (DCR) were 17.5% and 64.9%, respectively. Median progression free survival (PFS) and overall survival (OS) were 6.0 months (95% CI: 4.9-7.2) and 12.1 months (95% CI: 9.6-16.7). Median PFS was 6.9 months for CAPE-based regimens (95% CI: 5.0-18.4), 6.1 months (95% CI: 4.0-8.9) for CTX-based and 5.3 months (95% CI: 4.1-9.5) for VRL-based ones. Median OS was 18.2 months (95% CI: 9.1-NE) for CAPE-based regimens and 11.8 months for VRL- (95% CI: 9.3-16.7 and CTX-based ones (95%CI: 8.7-52.8). Tumour response, PFS and OS decreased proportionally in later lines.
Conclusion: This analysis represents the largest series of TNBC patients treated with mCHT in a real-life setting and provides further insights into the advantages of using this strategy even in this poor prognosis subpopulation.
Keywords: Capecitabine; Cyclophosphamide; Methotrexate; Metronomic chemotherapy; Triple-negative breast cancer; Vinorelbine.
© 2021. The Author(s).
Conflict of interest statement
Author Prof. Cazzaniga has a role as consultant/advisory role for: Pierre-Fabre, Roche; Novartis; Lilly; Celgene. Author Vallini I. declares that she has no conflict of interest; Author Dr Montagna has a role as consultant/advisory role for: Pierre-Fabre; Author Dr Amoroso declares that he has no conflict of interest; Author Dr Berardi declares that she has no conflict of interest; Author Dr Butera declares that she has no conflict of interest; Author Dr Cagossi declares that she has no conflict of interest; Author Dr Cavanna has a role as consultant/advisory role for: Astra Zeneca, Ipsen, Janssen; Author Dr Ciccarese declares that she has no conflict of interest;, Author Dr Cinieri has a role as consultant/advisory role for: Ely Lilly, Author Dr Cretella declares that she has no conflict of interest, Author Dr De Conciliis declares that he has no conflict of interest, Author Dr Febbraro declares that he has no conflict of interest, Author Dr Ferraù declares that he has no conflict of interest, Author Dr Ferzi declares that she has no conflict of interest, Author Dr Baldelli declares that he has no conflict of interest, Author Dr Fontana has a role as consultant/advisory role for: Novartis, Roche, Daiichi Sankyo, Pierre Phabre, Eli Lilly; Author Dr Gambaro declares that she has no conflict of interest, Author Dr Garrone declares that she has no conflict of interest, Author Dr Gebbia declares that he has no conflict of interest, Author Dr Generali has received a remuneration of 5.000 € and had a role as consultant/advisory role for: Novartis, FMI, Istituto Gentili, Roche, Pfizer, Ely Lilly, Author Dr Gianni declares that he has no conflict of interest, Author Dr Giovanardi declares that he has no conflict of interest,, Author Dr Grassadonia declares that he has no conflict of interest, Author Dr Leonardi declares that she has no conflict of interest, Author Dr Marchetti declares that he has no conflict of interest, Author Dr Sarti declares that she has no conflict of interest, Author Dr Musolino remuneration acting as consultant/advisory role for: Eisai, Ely Lilly, Seagen, Novartis, Roche; Author Dr Nicolini declares that he has no conflict of interest, Author Dr Putzu declares that he has no conflict of interest,, Author Dr Riccardi declares that he has no conflict of interest,, Author Dr Santini has a role as consultant/advisory role for: Novartis, Lilly, Eisai; Author Dr Saracchini declares that she has no conflict of interest,, Author Dr Sarobba declares that she has no conflict of interest,, Author Dr Schintu declares that she has no conflict of interest, Author Dr Scognamiglio declares that he has no conflict of interest, Author Dr Spadaro declares that he has no conflict of interest,. Author Dr Taverniti declares that he has no conflict of interest, Author Dr Toniolo declares that he has no conflict of interest,, Author Dr Tralongo declares that he has no conflict of interest, Author Dr Turletti declares that she has no conflict of interest, Author Dr Valenza declares that he has no conflict of interest,, Author Dr Valerio declares that he has no conflict of interest, Author Dr Vici Santini has a role as consultant/advisory role for: Roche, Pfizer, Novartis, Gentili, Eli Lilly; Author Clivio declares that he has no conflict of interest and Author Torri declares that he has no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous