Clinical characterization of Lassa fever: A systematic review of clinical reports and research to inform clinical trial design
- PMID: 34547033
- PMCID: PMC8486098
- DOI: 10.1371/journal.pntd.0009788
Clinical characterization of Lassa fever: A systematic review of clinical reports and research to inform clinical trial design
Abstract
Background: Research is urgently needed to reduce the morbidity and mortality of Lassa fever (LF), including clinical trials to test new therapies and to verify the efficacy and safety of the only current treatment recommendation, ribavirin, which has a weak clinical evidence base. To help establish a basis for the development of an adaptable, standardised clinical trial methodology, we conducted a systematic review to identify the clinical characteristics and outcomes of LF and describe how LF has historically been defined and assessed in the scientific literature.
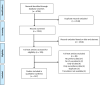
Methodology: Primary clinical studies and reports of patients with suspected and confirmed diagnosis of LF published in the peer-reviewed literature before 15 April 2021 were included. Publications were selected following a two-stage screening of abstracts, then full-texts, by two independent reviewers at each stage. Data were extracted, verified, and summarised using descriptive statistics.
Results: 147 publications were included, primarily case reports (36%), case series (28%), and cohort studies (20%); only 2 quasi-randomised studies (1%) were found. Data are mostly from Nigeria (52% of individuals, 41% of publications) and Sierra Leone (42% of individuals, 31% of publications). The results corroborate the World Health Organisation characterisation of LF presentation. However, a broader spectrum of presenting symptoms is evident, such as gastrointestinal illness and other nervous system and musculoskeletal disorders that are not commonly included as indicators of LF. The overall case fatality ratio was 30% in laboratory-confirmed cases (1896/6373 reported in 109 publications).
Conclusion: Systematic review is an important tool in the clinical characterisation of diseases with limited publications. The results herein provide a more complete understanding of the spectrum of disease which is relevant to clinical trial design. This review demonstrates the need for coordination across the LF research community to generate harmonised research methods that can contribute to building a strong evidence base for new treatments and foster confidence in their integration into clinical care.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- World Health Organisation. Lassa fever [12 October 2020]. Available from: https://www.who.int/health-topics/lassa-fever/#tab=tab_1.
-
- Nigeria Centre for Disease Control. National Guideline for Lassa Fever Case Management. 2018.
-
- Centers for Disease Control and Prevention. Lassa fever 2019 [12 October 2020]. Available from: https://www.cdc.gov/vhf/lassa/index.html.
-
- Kenmoe S, Tchatchouang S, Ebogo-Belobo JT, Ka’e AC, Mahamat G, Simo REG, et al. Systematic review and meta-analysis of the epidemiology of lassa virus in humans, rodents and other mammals in Sub-Saharan Africa. PLoS Neglected Tropical Diseases. 2020;14(8):1–29. doi: 10.1371/journal.pntd.0008589 . - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous