Effect of Slower vs Faster Intravenous Fluid Bolus Rates on Mortality in Critically Ill Patients: The BaSICS Randomized Clinical Trial
- PMID: 34547081
- PMCID: PMC8356145
- DOI: 10.1001/jama.2021.11444
Effect of Slower vs Faster Intravenous Fluid Bolus Rates on Mortality in Critically Ill Patients: The BaSICS Randomized Clinical Trial
Abstract
Importance: Slower intravenous fluid infusion rates could reduce the formation of tissue edema and organ dysfunction in critically ill patients; however, there are no data to support different infusion rates during fluid challenges for important outcomes such as mortality.
Objective: To determine the effect of a slower infusion rate vs control infusion rate on 90-day survival in patients in the intensive care unit (ICU).
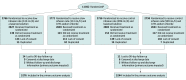
Design, setting, and participants: Unblinded randomized factorial clinical trial in 75 ICUs in Brazil, involving 11 052 patients requiring at least 1 fluid challenge and with 1 risk factor for worse outcomes were randomized from May 29, 2017, to March 2, 2020. Follow-up was concluded on October 29, 2020. Patients were randomized to 2 different infusion rates (reported in this article) and 2 different fluid types (balanced fluids or saline, reported separately).
Interventions: Patients were randomized to receive fluid challenges at 2 different infusion rates; 5538 to the slower rate (333 mL/h) and 5514 to the control group (999 mL/h). Patients were also randomized to receive balanced solution or 0.9% saline using a factorial design.
Main outcomes and measures: The primary end point was 90-day survival.
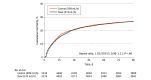
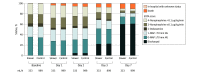
Results: Of all randomized patients, 10 520 (95.2%) were analyzed (mean age, 61.1 years [SD, 17.0 years]; 44.2% were women) after excluding duplicates and consent withdrawals. Patients assigned to the slower rate received a mean of 1162 mL on the first day vs 1252 mL for the control group. By day 90, 1406 of 5276 patients (26.6%) in the slower rate group had died vs 1414 of 5244 (27.0%) in the control group (adjusted hazard ratio, 1.03; 95% CI, 0.96-1.11; P = .46). There was no significant interaction between fluid type and infusion rate (P = .98).
Conclusions and relevance: Among patients in the intensive care unit requiring fluid challenges, infusing at a slower rate compared with a faster rate did not reduce 90-day mortality. These findings do not support the use of a slower infusion rate.
Trial registration: ClinicalTrials.gov Identifier: NCT02875873.
Conflict of interest statement
Figures
Comment in
-
Does crystalloid infusion rate really matter in critically ill patients?Anaesth Crit Care Pain Med. 2021 Dec;40(6):100982. doi: 10.1016/j.accpm.2021.100982. Epub 2021 Nov 9. Anaesth Crit Care Pain Med. 2021. PMID: 34767978 No abstract available.
-
Slower vs Faster Intravenous Fluid Bolus Rates and Mortality in Critically Ill Patients.JAMA. 2021 Dec 14;326(22):2331-2332. doi: 10.1001/jama.2021.18542. JAMA. 2021. PMID: 34905037 No abstract available.
-
In critically ill adults, IV fluid bolus infused at slower vs. faster rates did not differ for 90-d mortality.Ann Intern Med. 2022 Feb;175(2):JC23. doi: 10.7326/J21-0015. Epub 2022 Feb 1. Ann Intern Med. 2022. PMID: 35099988
References
-
- Hernández G, Ospina-Tascón GA, Damiani LP, et al. ; The ANDROMEDA SHOCK Investigators and the Latin America Intensive Care Network (LIVEN) . Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA. 2019;321(7):654-664. doi:10.1001/jama.2019.0071 - DOI - PMC - PubMed
-
- Dellinger RP, Levy MM, Carlet JM, et al. ; International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses; American College of Chest Physicians; American College of Emergency Physicians; Canadian Critical Care Society; European Society of Clinical Microbiology and Infectious Diseases; European Society of Intensive Care Medicine; European Respiratory Society; International Sepsis Forum; Japanese Association for Acute Medicine; Japanese Society of Intensive Care Medicine; Society of Critical Care Medicine; Society of Hospital Medicine; Surgical Infection Society; World Federation of Societies of Intensive and Critical Care Medicine . Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296-327. doi:10.1097/01.CCM.0000298158.12101.41 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

