Effect of School Integrated Pest Management or Classroom Air Filter Purifiers on Asthma Symptoms in Students With Active Asthma: A Randomized Clinical Trial
- PMID: 34547084
- PMCID: PMC8424475
- DOI: 10.1001/jama.2021.11559
Effect of School Integrated Pest Management or Classroom Air Filter Purifiers on Asthma Symptoms in Students With Active Asthma: A Randomized Clinical Trial
Abstract
Importance: School and classroom allergens and particles are associated with asthma morbidity, but the benefit of environmental remediation is not known.
Objective: To determine whether use of a school-wide integrated pest management (IPM) program or high-efficiency particulate air (HEPA) filter purifiers in the classrooms improve asthma symptoms in students with active asthma.
Design, setting, and participants: Factorial randomized clinical trial of a school-wide IPM program and HEPA filter purifiers in the classrooms was conducted from 2015 to 2020 (School Inner-City Asthma Intervention Study). There were 236 students with active asthma attending 41 participating urban elementary schools located in the Northeastern US who were randomized to IPM by school and HEPA filter purifiers by classroom. The date of final follow-up was June 20, 2020.
Interventions: The school-wide IPM program consisted of application of rodenticide, sealing entry points, trap placement, targeted cleaning, and brief educational handouts for school staff. Infestation was assessed every 3 months, with additional treatments as needed. Control schools received no IPM, cleaning, or education. Classroom portable HEPA filter purifiers were deployed and the filters were changed every 3 months. Control classrooms received sham HEPA filters that looked and sounded like active HEPA filter purifiers. Randomization was done independently (split-plot design), with matching by the number of enrolled students to ensure a nearly exact 1:1 student ratio for each intervention with 118 students randomized to each group. Participants, investigators, and those assessing outcomes were blinded to the interventions.
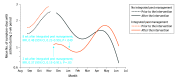
Main outcomes and measures: The primary outcome was the number of symptom-days with asthma during a 2-week period. Symptom-days were assessed every 2 months during the 10 months after randomization.
Results: Among the 236 students who were randomized (mean age, 8.1 [SD, 2.0] years; 113 [48%] female), all completed the trial. At baseline, the 2-week mean was 2.2 (SD, 3.9) symptom-days with asthma and 98% of the classrooms had detectable levels of mouse allergen. The results were pooled because there was no statistically significant difference between the 2 interventions (P = .18 for interaction). During a 2-week period, the mean was 1.5 symptom-days with asthma after use of the school-wide IPM program vs 1.9 symptom-days after no IPM across the school year (incidence rate ratio, 0.71 [95% CI, 0.38-1.33]), which was not statistically significantly different. During a 2-week period, the mean was 1.6 symptom-days with asthma after use of HEPA filter purifiers in the classrooms vs 1.8 symptom-days after use of sham HEPA filter purifiers across the school year (incidence rate ratio, 1.47 [95% CI, 0.79-2.75]), which was not statistically significantly different. There were no intervention-related adverse events.
Conclusions and relevance: Among children with active asthma, use of a school-wide IPM program or classroom HEPA filter purifiers did not significantly reduce symptom-days with asthma. However, interpretation of the study findings may need to consider allergen levels, particle exposures, and asthma symptoms at baseline.
Trial registration: ClinicalTrials.gov Identifier: NCT02291302.
Conflict of interest statement
Figures

Comment in
-
School Classrooms as Targets to Reduce Allergens and Improve Asthma.JAMA. 2021 Sep 7;326(9):816-817. doi: 10.1001/jama.2021.12322. JAMA. 2021. PMID: 34547097 No abstract available.
References
-
- Del-Rio-Navarro BE, Navarrete-Rodríguez EM, Berber A, Reyes-Noriega N, García-Marcos Álvarez L; Grupo GAN México, Grupo ISAAC México . The burden of asthma in an inner-city area: a historical review 10 years after Isaac. World Allergy Organ J. 2020;13(1):100092. doi:10.1016/j.waojou.2019.100092 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- K24 AI106822/AI/NIAID NIH HHS/United States
- P30 ES005605/ES/NIEHS NIH HHS/United States
- K23 ES023700/ES/NIEHS NIH HHS/United States
- K23 ES031663/ES/NIEHS NIH HHS/United States
- K23 HL150341/HL/NHLBI NIH HHS/United States
- U01 AI110397/AI/NIAID NIH HHS/United States
- K23 AI104780/AI/NIAID NIH HHS/United States
- P30 ES000002/ES/NIEHS NIH HHS/United States
- K23 AI143962/AI/NIAID NIH HHS/United States
- K23 AI106945/AI/NIAID NIH HHS/United States
- R01 ES030100/ES/NIEHS NIH HHS/United States
- UL1 TR000170/TR/NCATS NIH HHS/United States
- K23 AI123517/AI/NIAID NIH HHS/United States
- K12 HS022986/HS/AHRQ HHS/United States
- L30 HL143781/HL/NHLBI NIH HHS/United States

