Disease mechanisms of X-linked cone dystrophy caused by missense mutations in the red and green cone opsins
- PMID: 34547123
- PMCID: PMC8462070
- DOI: 10.1096/fj.202101066R
Disease mechanisms of X-linked cone dystrophy caused by missense mutations in the red and green cone opsins
Abstract
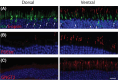
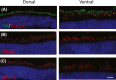
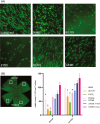
Cone photoreceptors are responsible for the visual acuity and color vision of the human eye. Red/green cone opsin missense mutations N94K, W177R, P307L, R330Q, and G338E have been identified in subjects with congenital blue cone monochromacy or color-vision deficiency. Studies on disease mechanisms due to these cone opsin mutations have been previously carried out exclusively in vitro, and the reported impairments were not always consistent. Here we expressed these mutants via AAV specifically in vivo in M-opsin knockout mouse cones to investigate their subcellular localization, the pathogenic effects on cone structure, function, and cone viability. We show that these mutations alter the M-opsin structure, function, and localization. N94K and W177R mutants appeared to be misfolded since they localized exclusively in cone inner segments and endoplasmic reticulum. In contrast, P307L, R330Q, and G338E mutants were detected predominately in cone outer segments. Expression of R330Q and G338E, but not P307L opsins, also partially restored expression and correct localization of cone PDE6α' and cone transducin γ and resulted in partial rescue of M-cone-mediated light responses. Expression of W177R and P307L mutants significantly reduced cone viability, whereas N94K, R330Q, and G338E were only modestly toxic. We propose that although the underlying biochemical and cellular defects caused by these mutants are distinct, they all seem to exhibit a dominant phenotype, resembling autosomal dominant retinitis pigmentosa associated with the majority of rhodopsin missense mutations. The understanding of the molecular mechanisms associated with these cone opsin mutants is fundamental to developing targeted therapies for cone dystrophy/dysfunction.
Keywords: blue cone monochromacy; cone dystrophy; cone opsin; disease mechanism; photoreceptors.
© 2021 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
WWH and the University of Florida have a financial interest in the use of AAV therapies, and WWH owns equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work.
Figures




References
-
- Curcio CA, Allen KA, Sloan KR, et al. Distribution and morphology of human cone photoreceptors stained with anti‐blue opsin. J Comp Neurol. 1991;312:610‐624. - PubMed
-
- Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497‐523. - PubMed
-
- Nathans J, Thomas D, Hogness DS. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986;232:193‐202. - PubMed
-
- Ayyagari R, Kakuk LE, Coats CL, et al. Bilateral macular atrophy in blue cone monochromacy (BCM) with loss of the locus control region (LCR) and part of the red pigment gene. Mol Vis. 1999;5:13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

