Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy
- PMID: 34547533
- PMCID: PMC8451978
- DOI: 10.1016/j.ajogmf.2021.100492
Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy
Abstract
Background: The exclusion of pregnant women from initial COVID-19 messenger RNA vaccine trials raised hesitancy regarding the benefits of vaccination for pregnant women, hence little is known about vaccines' efficacy in this population.
Objective: To determine the maternal-neonatal transplacental transfer of SARS-CoV-2 antibodies among vaccinated parturient women. A control group of COVID-19-recovered patients was included to compare the immunoglobulin G levels between vaccinated and recovered patients.
Study design: This is a prospective cohort study conducted in a single tertiary medical center in Israel between February and March 2021; parturient women vaccinated with the BNT162b2 messenger RNA vaccine during pregnancy were included and compared with COVID-19-recovered parturient women. SARS-CoV-2 immunoglobulin G antibodies were measured in maternal and cord sera, dried blood spot samples taken from newborns, and breast milk samples. The primary aim was to determine whether neonatal cord and dried blood spot samples were positive for SARS-CoV-2 antibodies and to evaluate the transfer ratio, defined as cord blood immunoglobulin G divided by maternal immunoglobulin G levels.
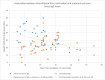
Results: The study included 64 vaccinated parturient women and 11 parturient women who had COVID-19 during pregnancy. All maternal blood sera samples and 98.3% of the cord blood sera samples were positive for SARS-Cov-2 immunoglobulin G with median concentrations of 26.1 (interquartile range, 22.0-39.7) and 20.2 (interquartile range, 12.7-29.0), respectively. Similarly, 96.4% of neonatal blood spot samples and all breast milk samples were positive for SARS-CoV-2 immunoglobulin G with median concentrations of 11.0 (interquartile range, 7.2-12.8) and 4.9 (interquartile range, 3.8-6.0), respectively. There was a significant positive correlation between maternal serum levels of SARS-CoV-2 immunoglobulin G and cord blood (r=0.483; P=.0001), neonatal blood spot (r=0.515; P=.004), and breast milk levels (r=0.396; P=.005) of SARS-CoV-2 immunoglobulin G. The median placental transfer ratio of SARS-COV-2 immunoglobulin G was 0.77. Comparison of vaccinated and recovered COVID-19 patients revealed significantly higher SARS-CoV-2 immunoglobulin G levels in maternal serum and cord blood among vaccinated women (P<.0001).
Conclusion: Our study demonstrated the efficient transfer of SARS-CoV-2 immunoglobulin G across the placenta in women, vaccinated with the BNT162b2 messenger RNA vaccine during pregnancy, to their neonates, with a positive correlation between maternal serum and cord blood antibody concentrations. In addition to maternal protection against COVID-19, the vaccine may also provide neonatal humoral immunity.
Keywords: COVID-19 vaccine; SARS-CoV-2 antibodies; antibodies transfer; neonatal immunity; pregnancy.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures


References
-
- American College of Obstetricians and Gynecologists. COVID-19 vaccination considerations for obstetrics-gynecologic care. 2020. Available at:https://www.acog.org/clinical/clinical-guidance/practice-advisory/articl.... Accessed October 1, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

