Sickle cell disease promotes sex-dependent pathological bone loss through enhanced cathepsin proteolytic activity in mice
- PMID: 34547771
- PMCID: PMC8905708
- DOI: 10.1182/bloodadvances.2021004615
Sickle cell disease promotes sex-dependent pathological bone loss through enhanced cathepsin proteolytic activity in mice
Abstract
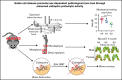
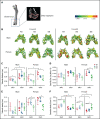
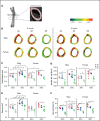
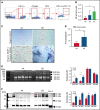
Sickle cell disease (SCD) is the most common hereditary blood disorder in the United States. SCD is frequently associated with osteonecrosis, osteoporosis, osteopenia, and other bone-related complications such as vaso-occlusive pain, ischemic damage, osteomyelitis, and bone marrow hyperplasia known as sickle bone disease (SBD). Previous SBD models have failed to distinguish the age- and sex-specific characteristics of bone morphometry. In this study, we use the Townes mouse model of SCD to assess the pathophysiological complications of SBD in both SCD and sickle cell trait. Changes in bone microarchitecture and bone development were assessed by using high-resolution quantitative micro-computed tomography and the three-dimensional reconstruction of femurs from male and female mice. Our results indicate that SCD causes bone loss and sex-dependent anatomical changes in bone. SCD female mice in particular are prone to trabecular bone loss, whereas cortical bone degradation occurs in both sexes. We also describe the impact of genetic knockdown of cathepsin K- and E-64-mediated cathepsin inhibition on SBD.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures


References
-
- Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4 Suppl):S512-S521. - PubMed
-
- Chiang EY, Frenette PS. Sickle cell vaso-occlusion. Hematol Oncol Clin North Am. 2005;19(5):771-784. - PubMed
-
- Aguilar C, Vichinsky E, Neumayr L. Bone and joint disease in sickle cell disease. Hematol Oncol Clin North Am. 2005;19(5):929-941. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

