Pathophysiology of Acute Respiratory Distress Syndrome and COVID-19 Lung Injury
- PMID: 34548132
- PMCID: PMC8162817
- DOI: 10.1016/j.ccc.2021.05.003
Pathophysiology of Acute Respiratory Distress Syndrome and COVID-19 Lung Injury
Abstract
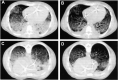
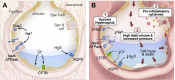
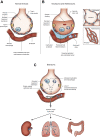
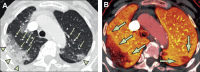
The pathophysiology of acute respiratory distress syndrome (ARDS) is marked by inflammation-mediated disruptions in alveolar-capillary permeability, edema formation, reduced alveolar clearance and collapse/derecruitment, reduced compliance, increased pulmonary vascular resistance, and resulting gas exchange abnormalities due to shunting and ventilation-perfusion mismatch. Mechanical ventilation, especially in the setting of regional disease heterogeneity, can propagate ventilator-associated injury patterns including barotrauma/volutrauma and atelectrauma. Lung injury due to the novel coronavirus SARS-CoV-2 resembles other causes of ARDS, though its initial clinical characteristics may include more profound hypoxemia and loss of dyspnea perception with less radiologically-evident lung injury, a pattern not described previously in ARDS.
Keywords: COVID-19; acute respiratory distress syndrome; pathophysiology.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures






References
-
- Villar J., Blanco J., Kacmarek R.M. Current incidence and outcome of the acute respiratory distress syndrome. Curr Opin Crit Care. 2016;22(1):1–6. - PubMed
-
- Taylor P.R., Martinez-Pomares L., Stacey M., et al. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. - PubMed
-
- Matthay M.A., Folkesson H.G., Verkman A. Salt and water transport across alveolar and distal airway epithelia in the adult lung. Am J Physiol. 1996;270(4):L487–L503. - PubMed
-
- Ware L.B., Matthay M.A. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163(6):1376–1383. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

