EGFR Inhibition Enhances the Cellular Uptake and Antitumor-Activity of the HER3 Antibody-Drug Conjugate HER3-DXd
- PMID: 34548332
- PMCID: PMC8732289
- DOI: 10.1158/0008-5472.CAN-21-2426
EGFR Inhibition Enhances the Cellular Uptake and Antitumor-Activity of the HER3 Antibody-Drug Conjugate HER3-DXd
Abstract
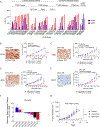
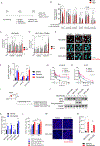
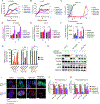
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) are the standard-of-care treatment for EGFR-mutant non-small cell lung cancers (NSCLC). However, most patients develop acquired drug resistance to EGFR TKIs. HER3 is a unique pseudokinase member of the ERBB family that functions by dimerizing with other ERBB family members (EGFR and HER2) and is frequently overexpressed in EGFR-mutant NSCLC. Although EGFR TKI resistance mechanisms do not lead to alterations in HER3, we hypothesized that targeting HER3 might improve efficacy of EGFR TKI. HER3-DXd is an antibody-drug conjugate (ADC) comprised of HER3-targeting antibody linked to a topoisomerase I inhibitor currently in clinical development. In this study, we evaluated the efficacy of HER3-DXd across a series of EGFR inhibitor-resistant, patient-derived xenografts and observed it to be broadly effective in HER3-expressing cancers. We further developed a preclinical strategy to enhance the efficacy of HER3-DXd through osimertinib pretreatment, which increased membrane expression of HER3 and led to enhanced internalization and efficacy of HER3-DXd. The combination of osimertinib and HER3-DXd may be an effective treatment approach and should be evaluated in future clinical trials in EGFR-mutant NSCLC patients. SIGNIFICANCE: EGFR inhibition leads to increased HER3 membrane expression and promotes HER3-DXd ADC internalization and efficacy, supporting the clinical development of the EGFR inhibitor/HER3-DXd combination in EGFR-mutant lung cancer.See related commentary by Lim et al., p. 18.
©2021 American Association for Cancer Research.
Figures


Comment in
-
Antibody-Drug Conjugates: A New Addition to the Treatment Landscape of EGFR-Mutant Non-Small Cell Lung Cancer.Cancer Res. 2022 Jan 1;82(1):18-20. doi: 10.1158/0008-5472.CAN-21-3481. Cancer Res. 2022. PMID: 34983785
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

