Impact of using spirometry on clinical decision making and quality of life in children: protocol for a single centre randomised controlled trial
- PMID: 34548360
- PMCID: PMC8458340
- DOI: 10.1136/bmjopen-2021-050974
Impact of using spirometry on clinical decision making and quality of life in children: protocol for a single centre randomised controlled trial
Abstract
Introduction: Although spirometry has been available for decades, it is underused in paediatric practice, other than in specialist clinics. This is unsurprising as there is limited evidence on the benefit of routine spirometry in improving clinical decision making and/or outcomes for children. We hypothesised that using spirometry for children being evaluated for respiratory diseases impacts on clinical decision making and/or improves patient-related outcome measures (PROMs) and/or quality of life (QoL), compared with not using spirometry.
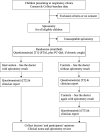
Methods and analysis: We are undertaking a randomised controlled trial (commenced in March 2020) that will include 106 children (aged 4-18 years) recruited from respiratory clinics at Queensland Children's Hospital, Australia. Inclusion criteria are able to perform reliable spirometry and a parent/guardian who can complete questionnaire(s). Children (1:1 allocation) are randomised to clinical medical review with spirometry (intervention group) or without spirometry (control group) within strata of consultation status (new/review), and cough condition (present/absent). The primary outcome is change in clinical decision making. The secondary outcomes are change in PROM scores, opinions regarding spirometry and degree of diagnosis certainty. Intergroup differences of these outcomes will be determined by χ2 test or unpaired t-test (or Mann-Whitney if not normally distributed). Change in outcomes within the control group after review of spirometry will also be assessed by McNemar's test or paired t-test/Wilcoxon signed-rank test.
Ethics and dissemination: The Human Research Ethics Committee of the Queensland Children's Hospital approved the study. The trial results will be disseminated through conference presentations, teaching avenues and publications.
Trial registration number: ACTRN12619001686190; Pre-results.
Keywords: change management; paediatric thoracic medicine; quality in health care.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources