Burosumab treatment in adults with X-linked hypophosphataemia: 96-week patient-reported outcomes and ambulatory function from a randomised phase 3 trial and open-label extension
- PMID: 34548383
- PMCID: PMC8458321
- DOI: 10.1136/rmdopen-2021-001714
Burosumab treatment in adults with X-linked hypophosphataemia: 96-week patient-reported outcomes and ambulatory function from a randomised phase 3 trial and open-label extension
Abstract
Objectives: To report the impact of burosumab on patient-reported outcomes (PROs) and ambulatory function in adults with X-linked hypophosphataemia (XLH) through 96 weeks.
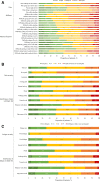
Methods: Adults diagnosed with XLH were randomised 1:1 in a double-blinded trial to receive subcutaneous burosumab 1 mg/kg or placebo every 4 weeks for 24 weeks (NCT02526160). Thereafter, all subjects received burosumab every 4 weeks until week 96. PROs were measured using the Western Ontario and the McMaster Universities Osteoarthritis Index (WOMAC), Brief Pain Inventory-Short Form (BPI-SF) and Brief Fatigue Inventory (BFI), and ambulatory function was measured with the 6 min walk test (6MWT).
Results: Subjects (N=134) were randomised to burosumab (n=68) or placebo (n=66) for 24 weeks. At baseline, subjects experienced pain, stiffness, and impaired physical and ambulatory function. At week 24, subjects receiving burosumab achieved statistically significant improvement in some BPI-SF scores, BFI worst fatigue (average and greatest) and WOMAC stiffness. At week 48, all WOMAC and BPI-SF scores achieved statistically significant improvement, with some WOMAC and BFI scores achieving meaningful and significant change from baseline. At week 96, all WOMAC, BPI-SF and BFI achieved statistically significant improvement, with selected scores in all measures also achieving meaningful change. Improvement in 6MWT distance and percent predicted were statistically significant at all time points from 24 weeks.
Conclusions: Adults with XLH have substantial burden of disease as assessed by PROs and 6MWT. Burosumab treatment improved phosphate homoeostasis and was associated with a steady and consistent improvement in PROs and ambulatory function.
Trial registration number: NCT02526160.
Keywords: healthcare; outcome assessment; patient reported outcome measures; therapeutics.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The following authors served as clinical investigators for one or more studies, including this trial, sponsored by Ultragenyx Pharmaceutical Inc. in partnership with Kyowa Kirin International: KB, AAP, MLB, TOC, HIC, MC-S, RKC, RE, YI, EAI, SI, NI, SJdB, MKJ, PK, RK, TK, RHL, FP, PP, SHR, YT, HT, TJW, H‑WY and KI. AAP and FP have also received honoraria from Ultragenyx Pharmaceutical for serving as an advisory board member or for lectures. PP has received research funding from Ultragenyx Pharmaceutical and is currently an employee of Ascendis Pharma. SHR has received grants from Kyowa Kirin International and Ultragenyx Pharmaceutical during the conduct of the study. During the conduct of the study, TJW also reports honoraria and travel support from Ultragenyx Pharmaceutical. AW and WS are employees of Kyowa Kirin International plc. AN and MN are employees of Chilli Consultancy and have received consultancy fees from Kyowa Kirin International to support the development of this manuscript and for projects outside this submitted work.
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
