Impact of Timing of Tocilizumab Use in Hospitalized Patients With SARS-CoV-2 Infection
- PMID: 34548407
- PMCID: PMC9993783
- DOI: 10.4187/respcare.08779
Impact of Timing of Tocilizumab Use in Hospitalized Patients With SARS-CoV-2 Infection
Abstract
Background: SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) continues to be a global challenge due to the lack of definitive treatment strategies. We sought to determine the efficacy of early administration of anti-interleukin 6 therapy in reducing hospital mortality and progression to mechanical ventilation.
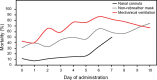
Methods: This was a retrospective chart review of 11,512 patients infected with SARS-CoV-2 who were admitted to a New York health system from March to May 2020. Tocilizumab was administered to subjects at the nasal cannula level of oxygen support to maintain an oxygen saturation of >88%. The Charlson comorbidity index was used as an objective assessment of the burden of comorbidities to predict 10-year mortality. The primary outcome of interest was hospital mortality. Secondary outcomes were progression to mechanical ventilation; the prevalence of venous thromboembolism and renal failure; and the change in C-reactive protein, D-dimer, and ferritin levels after tocilizumab administration. Propensity score matching by using a 1:2 protocol was used to match the tocilizumab and non-tocilizumab groups to minimize selection bias. The groups were matched on baseline demographic characteristics, including age, sex, and body mass index; Charlson comorbidity index score; laboratory markers, including ferritin, D-dimer, lactate dehydrogenase, and C-reactive protein values; and the maximum oxygen requirement at the time of tocilizumab administration. Mortality outcomes were evaluated based on the level of oxygen requirement and the day of hospitalization at the time of tocilizumab administration.
Results: The overall hospital mortality was significantly reduced in the tocilizumab group when tocilizumab was administered at the nasal cannula level (10.4% vs 22.0%; P = .002). In subjects who received tocilizumab at the nasal cannula level, the progression to mechanical ventilation was reduced versus subjects who were initially on higher levels of oxygen support (6.3% vs 18.7%; P < .001). There was no improvement in mortality when tocilizumab was given at the time of requiring non-rebreather, high-flow nasal cannula, noninvasive ventilator, or invasive ventilator.
Conclusions: Early use of anti-interleukin 6 therapy may be associated with improved hospital mortality and reduction in progression to more severe coronavirus disease 2019.
Keywords: COVID19 mortality; interlukin-6; tocilizumab.
Copyright © 2021 by Daedalus Enterprises.
Conflict of interest statement
The authors have disclosed no conflicts of interest.
Figures
Comment in
-
Timing of Tocilizumab Use and COVID-19.Respir Care. 2022 Mar;67(3):381-382. doi: 10.4187/respcare.09678. Respir Care. 2022. PMID: 35190482 No abstract available.
-
Impact of Timing of Tocilizumab Use in Hospitalized Patients With SARS-CoV-2 Infection.Respir Care. 2022 May;67(5):629-630. doi: 10.4187/respcare.10067. Respir Care. 2022. PMID: 35473844 No abstract available.
References
-
- World Health Organization. 2021. WHO Coronavirus (COVID-19) Dashboard, May 30, 2021, https://covid19.who.int. Accessed May 30, 2021.
-
- Tajbakhsh A, Gheibi Hayat SM, Taghizadeh H, Akbari A, Inabadi M, Savardashtaki A, et al. . COVID-19 adn cardiac injury: clinical manifestations biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev Anti Infect Ther 2021;19(3):345-357. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous