Prenylcysteine oxidase 1, an emerging player in atherosclerosis
- PMID: 34548610
- PMCID: PMC8455616
- DOI: 10.1038/s42003-021-02630-z
Prenylcysteine oxidase 1, an emerging player in atherosclerosis
Abstract
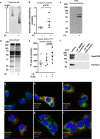
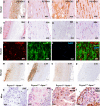
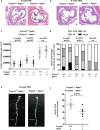
The research into the pathophysiology of atherosclerosis has considerably increased our understanding of the disease complexity, but still many questions remain unanswered, both mechanistically and pharmacologically. Here, we provided evidence that the pro-oxidant enzyme Prenylcysteine Oxidase 1 (PCYOX1), in the human atherosclerotic lesions, is both synthesized locally and transported within the subintimal space by proatherogenic lipoproteins accumulating in the arterial wall during atherogenesis. Further, Pcyox1 deficiency in Apoe-/- mice retards atheroprogression, is associated with decreased features of lesion vulnerability and lower levels of lipid peroxidation, reduces plasma lipid levels and inflammation. PCYOX1 silencing in vitro affects the cellular proteome by influencing multiple functions related to inflammation, oxidative stress, and platelet adhesion. Collectively, these findings identify the pro-oxidant enzyme PCYOX1 as an emerging player in atherogenesis and, therefore, understanding the biology and mechanisms of all functions of this unique enzyme is likely to provide additional therapeutic opportunities in addressing atherosclerosis.
© 2021. The Author(s).
Conflict of interest statement
We disclose the following European Patent application, under examination, EP15817414.4 entitled “Prenylcysteine oxidase 1 inhibitors for the prevention and/or treatment of oxidative stress-related degenerative diseases and prenylcysteine oxidase 1 as diagnostic marker”, applicant Centro Cardiologico Monzino IRCCS, inventors C.B., M.B., R.B, and S.B., with no financial competing interest. The remaining authors declare no competing interest.
Figures



Similar articles
-
Prenylcysteine Oxidase 1 (PCYOX1), a New Player in Thrombosis.Int J Mol Sci. 2022 Mar 4;23(5):2831. doi: 10.3390/ijms23052831. Int J Mol Sci. 2022. PMID: 35269975 Free PMC article.
-
Prenylcysteine Oxidase 1 Is a Key Regulator of Adipogenesis.Antioxidants (Basel). 2023 Feb 21;12(3):542. doi: 10.3390/antiox12030542. Antioxidants (Basel). 2023. PMID: 36978789 Free PMC article.
-
Pharmacological inhibition of histone deacetylase reduces NADPH oxidase expression, oxidative stress and the progression of atherosclerotic lesions in hypercholesterolemic apolipoprotein E-deficient mice; potential implications for human atherosclerosis.Redox Biol. 2020 Jan;28:101338. doi: 10.1016/j.redox.2019.101338. Epub 2019 Oct 4. Redox Biol. 2020. PMID: 31634818 Free PMC article.
-
Dual signaling evoked by oxidized LDLs in vascular cells.Free Radic Biol Med. 2017 May;106:118-133. doi: 10.1016/j.freeradbiomed.2017.02.006. Epub 2017 Feb 9. Free Radic Biol Med. 2017. PMID: 28189852 Review.
-
MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis.FASEB J. 2011 Aug;25(8):2515-27. doi: 10.1096/fj.11-181149. Epub 2011 Apr 20. FASEB J. 2011. PMID: 21507901 Review.
Cited by
-
The Proteome of Exosomes at Birth Predicts Insulin Resistance, Adrenarche and Liver Fat in Childhood.Int J Mol Sci. 2025 Feb 18;26(4):1721. doi: 10.3390/ijms26041721. Int J Mol Sci. 2025. PMID: 40004184 Free PMC article.
-
Methylprednisolone pulse-enhanced neutrophil extracellular trap formation in mice with imiquimod-induced lupus-like disease, resulting in ischaemia of the femoral head cartilage.Lupus Sci Med. 2023 Dec 28;10(2):e001042. doi: 10.1136/lupus-2023-001042. Lupus Sci Med. 2023. PMID: 38154828 Free PMC article.
-
Prenylcysteine oxidase 1 like protein is required for neutrophil bactericidal activities.Nat Commun. 2023 May 13;14(1):2761. doi: 10.1038/s41467-023-38447-z. Nat Commun. 2023. PMID: 37179332 Free PMC article.
-
Evolution, structure, and drug-metabolizing activity of mammalian prenylcysteine oxidases.J Biol Chem. 2024 Nov;300(11):107810. doi: 10.1016/j.jbc.2024.107810. Epub 2024 Sep 24. J Biol Chem. 2024. PMID: 39322016 Free PMC article.
-
Phenelzine-based probes reveal Secernin-3 is involved in thermal nociception.Mol Cell Neurosci. 2023 Jun;125:103842. doi: 10.1016/j.mcn.2023.103842. Epub 2023 Mar 15. Mol Cell Neurosci. 2023. PMID: 36924917 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

