Exploratory comparisons between different anti-mitotics in clinically-used drug combination in triple negative breast cancer
- PMID: 34548908
- PMCID: PMC8448514
- DOI: 10.18632/oncotarget.28068
Exploratory comparisons between different anti-mitotics in clinically-used drug combination in triple negative breast cancer
Erratum in
-
Correction: Exploratory comparisons between different anti-mitotics in clinically-used drug combination in triple negative breast cancer.Oncotarget. 2022 Sep 28;13:1068. doi: 10.18632/oncotarget.28266. eCollection 2022. Oncotarget. 2022. PMID: 36187557 Free PMC article.
Abstract
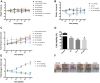
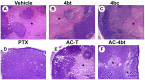
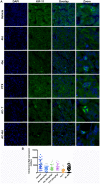
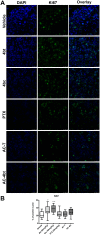
Triple-negative breast cancer (TNBC) constitutes a very aggressive type of breast cancer with few options of cytotoxic chemotherapy available for them. A chemotherapy regimen comprising of doxorubicin hydrochloride and cyclophosphamide, followed by paclitaxel, known as AC-T, is approved for usage as an adjuvant treatment for this type of breast cancer. In this study we aimed to elucidate the role of KIF11 in TNBC progression throughout its inhibition by two synthetic small molecules containing the DHPM core (dihydropyrimidin-2(1H)-ones or -thiones), with the hypothesis that these inhibitors could be an interesting option of antimitotic drug used alone or as adjuvant therapy in association with AC. For this purpose, we evaluated the efficacy of DHPMs used as monotherapy or in combination with doxorubicin and cyclophosphamide, in Balbc-nude mice bearing breast cancer induced by MDA-MB-231, having AC-T as positive control. Our data provide extensive evidence to demonstrate that KIF11 inhibitors showed pronounced antitumor activity, acting in key points of tumorigenesis and cancer progression in in vivo xenograft model of triple negative breast cancer, like down-regulation of KIF11 and ALDH1-A1. Moreover, they didn't show the classic peripheral neuropathy characterized by impaired mobility, as it is common with paclitaxel use. These results suggest that the use of a MAP inhibitor in breast cancer regimen treatment could be a promising strategy to keep antitumoral activity reducing the side effects.
Keywords: KIF11 inhibition; Kinesin Eg5; adjuvant treatment; breast cancer; cancer progression.
Copyright: © 2021 Guido et al.
Conflict of interest statement
CONFLICTS OF INTEREST Authors have no conflicts of interest to declare.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

