Understanding the Potential Impact of Different Drug Properties on Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Transmission and Disease Burden: A Modelling Analysis
- PMID: 34549260
- PMCID: PMC9402649
- DOI: 10.1093/cid/ciab837
Understanding the Potential Impact of Different Drug Properties on Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Transmission and Disease Burden: A Modelling Analysis
Abstract
Background: The public health impact of the coronavirus disease 2019 (COVID-19) pandemic has motivated a rapid search for potential therapeutics, with some key successes. However, the potential impact of different treatments, and consequently research and procurement priorities, have not been clear.
Methods: Using a mathematical model of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission, COVID-19 disease and clinical care, we explore the public-health impact of different potential therapeutics, under a range of scenarios varying healthcare capacity, epidemic trajectories; and drug efficacy in the absence of supportive care.
Results: The impact of drugs like dexamethasone (delivered to the most critically-ill in hospital and whose therapeutic benefit is expected to depend on the availability of supportive care such as oxygen and mechanical ventilation) is likely to be limited in settings where healthcare capacity is lowest or where uncontrolled epidemics result in hospitals being overwhelmed. As such, it may avert 22% of deaths in high-income countries but only 8% in low-income countries (assuming R = 1.35). Therapeutics for different patient populations (those not in hospital, early in the course of infection) and types of benefit (reducing disease severity or infectiousness, preventing hospitalization) could have much greater benefits, particularly in resource-poor settings facing large epidemics.
Conclusions: Advances in the treatment of COVID-19 to date have been focused on hospitalized-patients and predicated on an assumption of adequate access to supportive care. Therapeutics delivered earlier in the course of infection that reduce the need for healthcare or reduce infectiousness could have significant impact, and research into their efficacy and means of delivery should be a priority.
Keywords: COVID-19; SARS-CoV-2; epidemiology; modelling; therapeutics.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
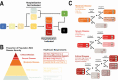



References
-
- Wibmer CK, Ayres F, Hermanus T, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Cold Spring Harbor Lab 2021. Available at: https://www.nature.com/articles/s41591-021-01285-x#citeas. Accessed 25 January 2021. - PubMed
-
- Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Cold Spring Harbor Lab 2021. Available at: https://www.nature.com/articles/s41586-021-03324-6. Accessed 25 January 2021. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

