Picrosirius Red Staining: Revisiting Its Application to the Qualitative and Quantitative Assessment of Collagen Type I and Type III in Tendon
- PMID: 34549650
- PMCID: PMC8504258
- DOI: 10.1369/00221554211046777
Picrosirius Red Staining: Revisiting Its Application to the Qualitative and Quantitative Assessment of Collagen Type I and Type III in Tendon
Abstract
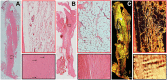
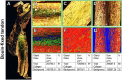
Collagen has a major role in the structural organization of tendons. Picrosirius red (PSR) staining viewed under polarized light microscopy is the standard method to evaluate the organization of collagen fibers in tissues. It is also used to distinguish between type I and type III collagen in tissue sections. However, accurate analysis and interpretation of PSR images are challenging because of technical factors and historical misconceptions. The aim of this study was to clarify whether collagen types I and III can be distinguished by PSR staining in rat Achilles tendons, using double immunohistochemistry as the positive control. Our findings showed that PSR staining viewed with polarized light microscopy was suitable for qualitative and quantitative assessment of total collagen but was not able to distinguish collagen types. We found it critical to use a polarizing microscope equipped with a rotating stage; tendon section orientation at 45° with respect to crossed polarizers was optimal for the qualitative and quantitative assessment of collagen organization. Immunohistochemistry was superior to PSR staining for detection of collagen type III. We also compared formalin and Bouin solution as fixatives. Both produced similar birefringence, but formalin-fixed tendons provided higher quality histological detail with both hematoxylin-eosin and immunostaining.
Keywords: Achilles tendon; Sirius red; collagen type; immunostaining; microscopy; polarization.
Conflict of interest statement
Figures


References
-
- Kirkendall DT, Garrett WE. Function and biomechanics of tendons. Scand J Med Sci Sports. 1997;7(2):62–6. - PubMed
-
- Wang JH. Mechanobiology of tendon. J Biomech. 2006; 39(9):1563–82. - PubMed
-
- Merkel JR, DiPaolo BR, Hallock GG, Rice DC. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med. 1988;187(4):493–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

