The Protective Effects of Live and Pasteurized Akkermansia muciniphila and Its Extracellular Vesicles against HFD/CCl4-Induced Liver Injury
- PMID: 34549998
- PMCID: PMC8557882
- DOI: 10.1128/Spectrum.00484-21
The Protective Effects of Live and Pasteurized Akkermansia muciniphila and Its Extracellular Vesicles against HFD/CCl4-Induced Liver Injury
Abstract
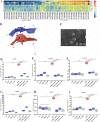
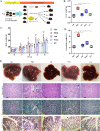
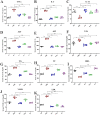
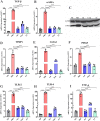
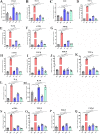
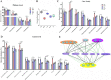
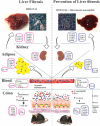
Akkermansia muciniphila, as a member of the gut microbiota, has been proposed as a next-generation probiotic. Liver fibrosis is the main determinant of liver dysfunction and mortality in patients with chronic liver disease. In this study, we aimed to determine the beneficial effects of live and pasteurized A. muciniphila and its extracellular vesicles (EVs) on the prevention of liver fibrosis. The response of hepatic stellate cells (HSCs) to live and pasteurized A. muciniphila and its EVs was examined in quiescent, lipopolysaccharide (LPS)-activated LX-2 cells. Liver fibrosis was induced in 8-week-old C57BL/6 mice, using a high-fat diet (HFD) and carbon tetrachloride (CCl4) administration for 4 weeks. The mice were concomitantly treated via oral gavage with three forms of bacteria. The relative expression of different fibrosis and inflammatory markers was assessed in the tissues. Histological markers, serum biochemical parameters, and cytokine production were also analyzed, and their correlations with the relative abundance of targeted fecal bacteria were examined. All A. muciniphila preparations exhibited protective effects against HSC activation; however, EVs showed the greatest activity in HSC regression. Oral gavage with A. muciniphila ameliorated the serum biochemical and inflammatory cytokines and improved liver and colon histopathological damages. The relative expression of fibrosis and inflammatory biomarkers was substantially attenuated in the tissues of all treated mice. The composition of targeted stool bacteria in the live A. muciniphila group was clearly different from that in the fibrosis group. This study indicated that A. muciniphila and its derivatives could successfully protect against HFD/CCl4-induced liver injury. However, further studies are needed to prove the beneficial effects of A. muciniphila on the liver. IMPORTANCE Akkermansia muciniphila, as a member of the gut microbiota, has been proposed as a next-generation probiotic. Liver fibrosis is the main determinant of liver dysfunction and mortality in patients with chronic liver disease. In this study, we aimed to determine the beneficial effects of live and pasteurized A. muciniphila and its extracellular vesicles (EVs) on the prevention of liver fibrosis. The results of the present study indicated that oral administration of live and pasteurized A. muciniphila and its EVs could normalize the fecal targeted bacteria composition, improve the intestinal permeability, modulate inflammatory responses, and subsequently prevent liver injury in HFD/CCl4-administered mice. Following the improvement of intestinal and liver histopathology, HFD/CCl4-induced kidney damage and adipose tissue inflammation were also ameliorated by different A. muciniphila treatments.
Keywords: Akkermansia muciniphila; extracellular vesicles; hepatic stellate cells; intestinal bacteria; liver fibrosis.
Figures
References
-
- Tanaka K, Takahashi H, Hyogo H, Ono M, Oza N, Kitajima Y, Kawanaka M, Chayama K, Saibara T, Anzai K, Eguchi Y. 2019. Epidemiological survey of hemoglobin A1c and liver fibrosis in a general population with non‐alcoholic fatty liver disease. Hepatol Res 49:296–303. doi: 10.1111/hepr.13282. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

