Molecular mechanisms of endomembrane trafficking in plants
- PMID: 34550393
- PMCID: PMC8773984
- DOI: 10.1093/plcell/koab235
Molecular mechanisms of endomembrane trafficking in plants
Abstract
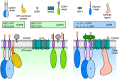
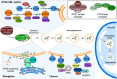
Endomembrane trafficking is essential for all eukaryotic cells. The best-characterized membrane trafficking organelles include the endoplasmic reticulum (ER), Golgi apparatus, early and recycling endosomes, multivesicular body, or late endosome, lysosome/vacuole, and plasma membrane. Although historically plants have given rise to cell biology, our understanding of membrane trafficking has mainly been shaped by the much more studied mammalian and yeast models. Whereas organelles and major protein families that regulate endomembrane trafficking are largely conserved across all eukaryotes, exciting variations are emerging from advances in plant cell biology research. In this review, we summarize the current state of knowledge on plant endomembrane trafficking, with a focus on four distinct trafficking pathways: ER-to-Golgi transport, endocytosis, trans-Golgi network-to-vacuole transport, and autophagy. We acknowledge the conservation and commonalities in the trafficking machinery across species, with emphasis on diversity and plant-specific features. Understanding the function of organelles and the trafficking machinery currently nonexistent in well-known model organisms will provide great opportunities to acquire new insights into the fundamental cellular process of membrane trafficking.
© American Society of Plant Biologists 2021. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures



Comment in
-
Back to the roots: A focus on plant cell biology.Plant Cell. 2022 Jan 20;34(1):1-3. doi: 10.1093/plcell/koab278. Plant Cell. 2022. PMID: 34755878 Free PMC article. No abstract available.
References
-
- Adolf F, Rhiel M, Hessling B, Gao Q, Hellwig A, Béthune J, Wieland FT (2019) Proteomic profiling of mammalian COPII and COPI vesicles. Cell Rep 26: 250–265 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

