A Systematic Evaluation of IgM and IgG Antibody Assay Accuracy in Diagnosing Acute Zika Virus Infection in Brazil: Lessons Relevant to Emerging Infections
- PMID: 34550810
- PMCID: PMC8601218
- DOI: 10.1128/JCM.02893-20
A Systematic Evaluation of IgM and IgG Antibody Assay Accuracy in Diagnosing Acute Zika Virus Infection in Brazil: Lessons Relevant to Emerging Infections
Abstract
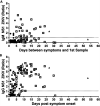
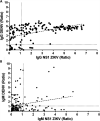
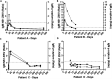
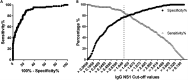
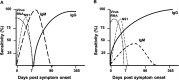
Accurate diagnostics underpin effective public health responses to emerging viruses. For viruses, such as Zika virus (ZIKV), where the viremia clears quickly, antibody-based (IgM or IgG) diagnostics are recommended for patients who present 7 days after symptom onset. However, cross-reactive antibody responses can complicate test interpretation among populations where closely related viruses circulate. We examined the accuracy (proportion of samples correctly categorized as Zika positive or negative) for antibody-based diagnostics among Brazilian residents (Rio de Janeiro) during the ZIKV outbreak. Four ZIKV enzyme-linked immunosorbent assays (ELISAs; IgM and IgG Euroimmun, IgM Novagnost, and CDC MAC), two dengue ELISAs (IgM and IgG Panbio), and the ZIKV plaque reduction neutralization test (PRNT) were evaluated. Positive samples were ZIKV PCR confirmed clinical cases collected in 2015-2016 (n = 169); negative samples (n = 236) were collected before ZIKV was present in Brazil (≤2013). Among serum samples collected ≥7 days from symptom onset, PRNT exhibited the highest accuracy (93.7%), followed by the Euroimmun IgG ELISA (77.9%). All IgM assays exhibited lower accuracy (<75%). IgG was detected more consistently than IgM among ZIKV cases using Euroimmun ELISAs (68% versus 22%). Anti-dengue virus IgM ELISA was positive in 41.1% of confirmed ZIKV samples tested. The Euroimmun IgG assay, although misdiagnosing 22% of samples, provided the most accurate ELISA. Anti-ZIKV IgG was detected more reliably than IgM among ZIKV patients, suggesting a secondary antibody response to assay antigens following ZIKV infection. Antibody ELISAs need careful evaluation in their target population to optimize use and minimize misdiagnosis, prior to widespread deployment, particularly where related viruses cocirculate.
Keywords: Zika antibody assays; Zika virus; diagnosis; immunoassays; plaque reduction neutralization tests (PRNTs); serology; serology evaluation.
Figures


Similar articles
-
Evaluation of Euroimmun Anti-Zika Virus IgM and IgG Enzyme-Linked Immunosorbent Assays for Zika Virus Serologic Testing.J Clin Microbiol. 2017 Aug;55(8):2462-2471. doi: 10.1128/JCM.00442-17. Epub 2017 May 31. J Clin Microbiol. 2017. PMID: 28566316 Free PMC article.
-
Serologic Testing for Zika Virus: Comparison of Three Zika Virus IgM-Screening Enzyme-Linked Immunosorbent Assays and Initial Laboratory Experiences.J Clin Microbiol. 2017 Jul;55(7):2127-2136. doi: 10.1128/JCM.00580-17. Epub 2017 Apr 26. J Clin Microbiol. 2017. PMID: 28446573 Free PMC article.
-
Evaluation of eight commercial Zika virus IgM and IgG serology assays for diagnostics and research.PLoS One. 2021 Jan 26;16(1):e0244601. doi: 10.1371/journal.pone.0244601. eCollection 2021. PLoS One. 2021. PMID: 33497414 Free PMC article.
-
Comparison of measles IgG enzyme immunoassays (EIA) versus plaque reduction neutralization test (PRNT) for measuring measles serostatus: a systematic review of head-to-head analyses of measles IgG EIA and PRNT.BMC Infect Dis. 2023 May 31;23(1):367. doi: 10.1186/s12879-023-08199-8. BMC Infect Dis. 2023. PMID: 37259032 Free PMC article.
-
Challenges for molecular and serological ZIKV infection confirmation.Childs Nerv Syst. 2018 Jan;34(1):79-84. doi: 10.1007/s00381-017-3641-5. Epub 2017 Nov 6. Childs Nerv Syst. 2018. PMID: 29110196 Review.
Cited by
-
Vaccine-induced T cell responses control Orthoflavivirus challenge infection without neutralizing antibodies in humans.Nat Microbiol. 2025 Feb;10(2):374-387. doi: 10.1038/s41564-024-01903-7. Epub 2025 Jan 10. Nat Microbiol. 2025. PMID: 39794472 Free PMC article. Clinical Trial.
-
Trends in ELISA-Based Flavivirus IgG Serosurveys: A Systematic Review.Trop Med Infect Dis. 2023 Apr 13;8(4):224. doi: 10.3390/tropicalmed8040224. Trop Med Infect Dis. 2023. PMID: 37104349 Free PMC article. Review.
-
Use of Envelope Domain III Protein for the Detection of IgG Type Antibodies Specific to Zika Virus by Indirect ELISA.Diagnostics (Basel). 2023 Jan 26;13(3):462. doi: 10.3390/diagnostics13030462. Diagnostics (Basel). 2023. PMID: 36766567 Free PMC article.
-
Serological Evidence of Widespread Zika Transmission across the Philippines.Viruses. 2021 Jul 23;13(8):1441. doi: 10.3390/v13081441. Viruses. 2021. PMID: 34452307 Free PMC article.
-
Production of Plant-Derived Japanese Encephalitis Virus Multi-Epitope Peptide in Nicotiana benthamiana and Immunological Response in Mice.Int J Mol Sci. 2023 Jul 19;24(14):11643. doi: 10.3390/ijms241411643. Int J Mol Sci. 2023. PMID: 37511402 Free PMC article.
References
-
- Centers for Disease Control and Prevention. 2018. Zika travel information: Traveler's health Centers for Disease Control and Prevention, Atlanta, GA.
-
- World Health Organization. 2019. Zika epidemiology update: Zika virus disease. World Health Organization, Geneva, Switzerland.
-
- World Health Organization. 2019. Zika epidemiology update. World Health Organization, Geneva, Switzerland.
-
- Sandra Giron FF, Decoppet A, Cadiou B, Travaglini T, Thirion L, Duran G, Jeannin C, L’Ambert G, Grard G, Noël H, Fournet N, Auzet-Caillaud M, Zandotti C, Aboukaïs S, Chaud P, Guedj S, Hamouda L, Naudot X, Ovize A, Lazarus C, de Valk H, Paty M-C, Leparc-Goffart I. 2019. Vector-borne transmission of Zika virus in Europe, southern France, August 2019. Eurosurveillance 24. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

