Implementation of an Anticoagulation Practice Guideline for COVID-19 via a Clinical Decision Support System in a Large Academic Health System and Its Evaluation: Observational Study
- PMID: 34550900
- PMCID: PMC8604256
- DOI: 10.2196/30743
Implementation of an Anticoagulation Practice Guideline for COVID-19 via a Clinical Decision Support System in a Large Academic Health System and Its Evaluation: Observational Study
Abstract
Background: Studies evaluating strategies for the rapid development, implementation, and evaluation of clinical decision support (CDS) systems supporting guidelines for diseases with a poor knowledge base, such as COVID-19, are limited.
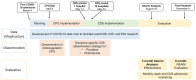
Objective: We developed an anticoagulation clinical practice guideline (CPG) for COVID-19, which was delivered and scaled via CDS across a 12-hospital Midwest health care system. This study represents a preplanned 6-month postimplementation evaluation guided by the RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance) framework.
Methods: The implementation outcomes evaluated were reach, adoption, implementation, and maintenance. To evaluate effectiveness, the association of CPG adherence on hospital admission with clinical outcomes was assessed via multivariable logistic regression and nearest neighbor propensity score matching. A time-to-event analysis was conducted. Sensitivity analyses were also conducted to evaluate the competing risk of death prior to intensive care unit (ICU) admission. The models were risk adjusted to account for age, gender, race/ethnicity, non-English speaking status, area deprivation index, month of admission, remdesivir treatment, tocilizumab treatment, steroid treatment, BMI, Elixhauser comorbidity index, oxygen saturation/fraction of inspired oxygen ratio, systolic blood pressure, respiratory rate, treating hospital, and source of admission. A preplanned subgroup analysis was also conducted in patients who had laboratory values (D-dimer, C-reactive protein, creatinine, and absolute neutrophil to absolute lymphocyte ratio) present. The primary effectiveness endpoint was the need for ICU admission within 48 hours of hospital admission.
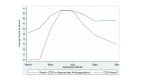
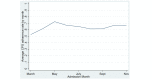
Results: A total of 2503 patients were included in this study. CDS reach approached 95% during implementation. Adherence achieved a peak of 72% during implementation. Variation was noted in adoption across sites and nursing units. Adoption was the highest at hospitals that were specifically transformed to only provide care to patients with COVID-19 (COVID-19 cohorted hospitals; 74%-82%) and the lowest in academic settings (47%-55%). CPG delivery via the CDS system was associated with improved adherence (odds ratio [OR] 1.43, 95% CI 1.2-1.7; P<.001). Adherence with the anticoagulation CPG was associated with a significant reduction in the need for ICU admission within 48 hours (OR 0.39, 95% CI 0.30-0.51; P<.001) on multivariable logistic regression analysis. Similar findings were noted following 1:1 propensity score matching for patients who received adherent versus nonadherent care (21.5% vs 34.3% incidence of ICU admission within 48 hours; log-rank test P<.001).
Conclusions: Our institutional experience demonstrated that adherence with the institutional CPG delivered via the CDS system resulted in improved clinical outcomes for patients with COVID-19. CDS systems are an effective means to rapidly scale a CPG across a heterogeneous health care system. Further research is needed to investigate factors associated with adherence at low and high adopting sites and nursing units.
Keywords: COVID-19; RE-AIM; anticoagulation; clinical decision support; clinical practice guideline; evidence-based practice; implementation science.
©Surbhi Shah, Sean Switzer, Nathan D Shippee, Pamela Wogensen, Kathryn Kosednar, Emma Jones, Deborah L Pestka, Sameer Badlani, Mary Butler, Brittin Wagner, Katie White, Joshua Rhein, Bradley Benson, Mark Reding, Michael Usher, Genevieve B Melton, Christopher James Tignanelli. Originally published in JMIR Medical Informatics (https://medinform.jmir.org), 18.11.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Similar articles
-
A pragmatic, stepped-wedge, hybrid type II trial of interoperable clinical decision support to improve venous thromboembolism prophylaxis for patients with traumatic brain injury.Implement Sci. 2024 Aug 5;19(1):57. doi: 10.1186/s13012-024-01386-4. Implement Sci. 2024. PMID: 39103955 Free PMC article.
-
Management of Renin-Angiotensin-Aldosterone System blockade in patients admitted to hospital with confirmed coronavirus disease (COVID-19) infection (The McGill RAAS-COVID- 19): A structured summary of a study protocol for a randomized controlled trial.Trials. 2021 Feb 5;22(1):115. doi: 10.1186/s13063-021-05080-4. Trials. 2021. PMID: 33546734 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Impact of summer programmes on the outcomes of disadvantaged or 'at risk' young people: A systematic review.Campbell Syst Rev. 2024 Jun 13;20(2):e1406. doi: 10.1002/cl2.1406. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38873396 Free PMC article. Review.
-
Clinical decision support systems for chronic obstructive pulmonary disease (COPD) in hospitals: A systematic review.Digit Health. 2023 Dec 10;9:20552076231219107. doi: 10.1177/20552076231219107. eCollection 2023 Jan-Dec. Digit Health. 2023. PMID: 38089165 Free PMC article. Review.
Cited by
-
Selection and Implementation of Virtual Scribe Solutions to Reduce Documentation Burden: A Mixed Methods Pilot.AMIA Jt Summits Transl Sci Proc. 2024 May 31;2024:230-238. eCollection 2024. AMIA Jt Summits Transl Sci Proc. 2024. PMID: 38827085 Free PMC article.
-
Differences in quality of anticoagulation care delivery according to ethnoracial group in the United States: A scoping review.J Thromb Thrombolysis. 2024 Aug;57(6):1076-1091. doi: 10.1007/s11239-024-02991-2. Epub 2024 May 11. J Thromb Thrombolysis. 2024. PMID: 38733515 Free PMC article.
-
A scoping review, novel taxonomy and catalogue of implementation frameworks for clinical decision support systems.BMC Med Inform Decis Mak. 2024 Nov 1;24(1):323. doi: 10.1186/s12911-024-02739-1. BMC Med Inform Decis Mak. 2024. PMID: 39487462 Free PMC article.
-
A pragmatic, stepped-wedge, hybrid type II trial of interoperable clinical decision support to improve venous thromboembolism prophylaxis for patients with traumatic brain injury.Implement Sci. 2024 Aug 5;19(1):57. doi: 10.1186/s13012-024-01386-4. Implement Sci. 2024. PMID: 39103955 Free PMC article.
-
Leveraging Dual Usability Methods to Evaluate Clinical Decision Support Among Patients With Traumatic Brain Injury: Mixed Methods Study.JMIR Hum Factors. 2025 Jul 30;12:e60268. doi: 10.2196/60268. JMIR Hum Factors. 2025. PMID: 40737617 Free PMC article.
References
-
- Mei H, Luo L, Hu Y. Thrombocytopenia and thrombosis in hospitalized patients with COVID-19. J Hematol Oncol. 2020 Dec 01;13(1):161–161. doi: 10.1186/s13045-020-01003-z. https://jhoonline.biomedcentral.com/articles/10.1186/s13045-020-01003-z 10.1186/s13045-020-01003-z - DOI - DOI - PMC - PubMed
-
- Atallah B, Mallah S, AlMahmeed W. Anticoagulation in COVID-19. Eur Heart J Cardiovasc Pharmacother. 2020 Jul 01;6(4):260–261. doi: 10.1093/ehjcvp/pvaa036. http://europepmc.org/abstract/MED/32352517 5827239 - DOI - PMC - PubMed
-
- Klok F, Kruip M, van der Meer N, Arbous M, Gommers D, Kant K, Kaptein F, van Paassen J, Stals M, Huisman M, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 Jul;191:145–147. doi: 10.1016/j.thromres.2020.04.013. http://europepmc.org/abstract/MED/32291094 S0049-3848(20)30120-1 - DOI - PMC - PubMed
-
- Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian CD, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GY, Global COVID-19 Thrombosis Collaborative Group‚ Endorsed by the ISTH‚ NATF‚ ESVM‚the IUA‚ Supported by the ESC Working Group on Pulmonary CirculationRight Ventricular Function COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020 Jun 16;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. https://linkinghub.elsevier.com/retrieve/pii/S0735-1097(20)35008-7 S0735-1097(20)35008-7 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

