Daratumumab for immune thrombotic thrombocytopenic purpura
- PMID: 34551063
- PMCID: PMC8945322
- DOI: 10.1182/bloodadvances.2021005124
Daratumumab for immune thrombotic thrombocytopenic purpura
Abstract
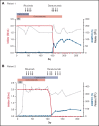
Immune thrombotic thrombocytopenic purpura (iTTP) is a life-threatening thrombotic microangiopathy. It is caused by a severe ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 motifs, 13) deficiency due to circulating autoantibodies, and is associated with significant morbidity and mortality. Current treatment options include plasma exchange, immunosuppression, and caplacizumab. When remission is achieved, the risk of relapse is high, especially in patients with persistent ADAMTS13 deficiency. We report the eradication of persistent ADAMTS13 inhibitory autoantibodies and restoration of normal ADAMTS13 activity using the anti-CD38 antibody daratumumab in two patients with iTTP. One patient had a frequently relapsing course, and the other a treatment-refractory first episode. There were no relevant adverse drug reactions.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures
References
-
- Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836-2846. - PubMed
-
- Mazepa MA, Masias C, Chaturvedi S. How targeted therapy disrupts the treatment paradigm for acquired TTP: the risks, benefits, and unknowns. Blood. 2019;134(5):415-420. - PubMed
-
- Cuker A, Cataland SR, Coppo P, et al. . Redefining outcomes in immune TTP: an international working group consensus report. Blood. 2021; 137(14):1855-1861. - PubMed
-
- Kremer Hovinga JA, Vesely SK, Terrell DR, Lämmle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115(8):1500-1511, quiz 1662. - PubMed
-
- Peyvandi F, Scully M, Kremer Hovinga JA, et al. ; TITAN Investigators . Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374(6):511-522. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

